RISEK INSTA Sachet
ក្រុមហ៊ុនផលិតឱសថ:
Getz Pharma(Pvt.) Limited, Pakistan.
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
ALLIANCE PHARMA CAMBODGE


- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
1. RISEK INSTA Powder for Oral Suspension 20mg:
Omeprazole 20mg, Sodium bicarbonate 1680mg
2. RISEK INSTA Powder for Oral Suspension 40mg:
Omeprazole 40mg, Sodium bicarbonate 1680mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
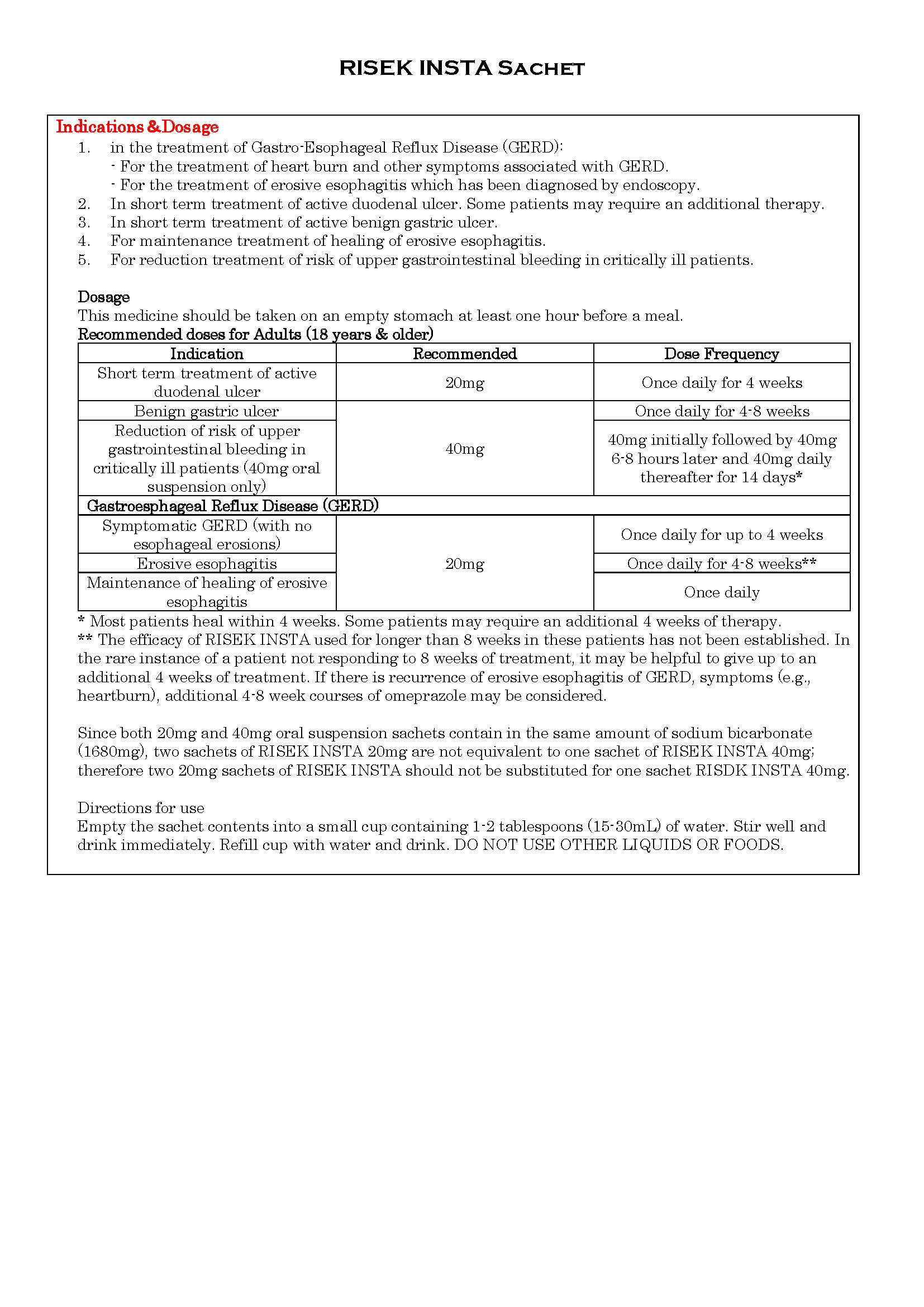
-
ហាមប្រើ
- Omeprazole is contraindicated in patients with known hypersensitivity to any component of the formulation.
- Sodium bicarbonate is contraindicated with metabolic alkalosis and hypocalcemia.
-
ផលរំខាន
Body as a whole
Allergic reactions, including, rarely anaphylaxis, fever, pain, fatigue, malaise and abdominal swelling.
Cardiovascular
Chest pain or angina, tachycardia, bradycardia, palpitation, elevated blood pressure, and peripheral edema.
Gastrointestinal
Pancreatitis (sometimes fatal), anorexia, irritable colon, flatulence, fecal discoloration, esophageal candidiasis, mucosal atrophy of the tongue, dry mouth, stomatitis. During treatment with omeprazole, gastric fundic gland polyps have been noted rarely. These polyps are benign and appear to be reversible when treatment is discontinued.
Hepatic
Mild and rarely, marked elevations of liver function tests [ALT(SGPT), AST(SGOT), ɤ-glutamyl transpeptidase, alkaline phosphatase, and bilirubin (jaundice)]. In rare instances, over liver disease has occurred, including hepatocellular, cholestatic, or mixed hepatitis, liver necrosis (sometimes fatal), hepatic failure (sometimes fatal), and hepatic encephalopathy.
Metabolic/ Nutritional
Hyponatremia, hypoglycemia, and weight gain.
Musculoskeletal
Muscle cramps, myalgia, muscle weakness, joint pain, and leg pain.
Nervous System/Psychiatric
Psychic disturbances including depression, agitation, aggression, hallucinations, confusion, insomnia, nervousness, tremors, apathy, somnolence, anxiety, dream abnormalities, vertigo, paresthesia and hemifacial dysesthesia.
Respiratory
Epistaxis, pharyngeal pain.
Skin
Rash and rarely, cases of severe generalized skin reactions including toxic epidermal necrolysis (TEN; sometimes fatal), Stevens-Johnson syndrome, and erythema multiforme (some severe); purpura and/or petechiae (sometimes with rechallenge); skin inflammation, urticaria, angioedema, pruritus, photosensitivity, alopecia, dry skin, and hyperhidrosis.
Special Senses
Tinnitus, taste perversion.
Ocular
Blurred vision, ocular irritation, dry eye syndrome, optic atrophy, anterior ischemic optic neuropathy, optic neuritis and double vision.
Urogenital
Interstitial nephritis (sometimes with positive rechallenge), urinary tract infection, microscopic pyuria, urinary frequency, elevated serum creatinine, proteinuria, hematuria, glycosuria, testicular pain, and gynecomastia.
Hematologic
Rare instances of pancytopenia, agranulocytosis (sometimes fatal), thrombocytopenia, neutropenia, leukopenia, anemia, leucocytosis, and hemolytic anemia have been reported.
Additional adverse reactions that could be caused by sodium bicarbonate include metabolic alkalosis, seizures, and tetany.
-
អន្តរប្រតិកម្ម
- diazepam, warfarin and phenytoin, drugs that are metabolized by oxidation in the liver, drugs metabolized via the cytochrome P-450 system (e.g., cyclosporine, Disulfiram, benzodiazepines).
- drugs where gastric pH is an important determinant of their bioavailability (e.g., ketoconazole, ampicillin esters, and iron salts).
- atazanavir
- tacrolimus
- clarithromycin
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
There are no adequate and well controlled studies on the use of omeprazole in pregnant women. Omeprazole should be used during pregnancy only if the potential benefit to pregnant women justifies the potential risk to the fetus.
Nursing Mothers
Omeprazole is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from omeprazole; a decision should be made whether, to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In addition, sodium bicarbonate should be used with caution in nursing mothers.
-
ការប្រុងប្រយ័ត្នជាពិសេស
General:
- Symptomatic response to therapy with omeprazole does not preclude the presence of gastric malignancy.
- Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole.
- Omeprazole powder for oral suspension contains sodium in the form of sodium bicarbonate. This should be taken into consideration for patients on a sodium-restricted diet.
- Sodium bicarbonate should be used with caution in patients with Barter’s syndrome, hypokalemia, respiratory alkalosis, and problems with acid-bases balance. Long-term administration of bicarbonate with calcium or milk can cause milk-alkali syndrome.
-
សកម្មភាពឱសថ
RISEK INSTA (Omeprazole + Sodium bicarbonate) is a combination of omeprazole, a proton-pump inhibitor and sodium bicarbonate, an antacid. Risek insta contains immediate-release formulation of omeprazole and sodium bicarbonate. Sodium bicarbonate raises the gastric pH and thus protect omeprazole from acid degradation.
Omeprazole is substituted benzimidazole 5-methoxy-
Omeprazole reduces gastric acid secretion through a unique mechanism of action. Omeprazole belongs to a class of anti-secretory compounds – the substituted benzimidazoles that do not exhibit anti-cholinergic or H2 histamine antagonistic properties. It inhibits secretion of gastric acid by irreversibly blocking the enzyme system of hydrogen/potassium adenosine triphosphatase (H+/K+ATPase), the proton pump of the gastric perinatal cell. This effect is dose-related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
