CLAVENTIN Caplet
ក្រុមហ៊ុនផលិតឱសថ:
PT DANKOS FARMA, Indonesia
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
ALLIANCE PHARMA CAMBODGE

- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Amoxicillin 500mg, Clavulanic acid 125mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
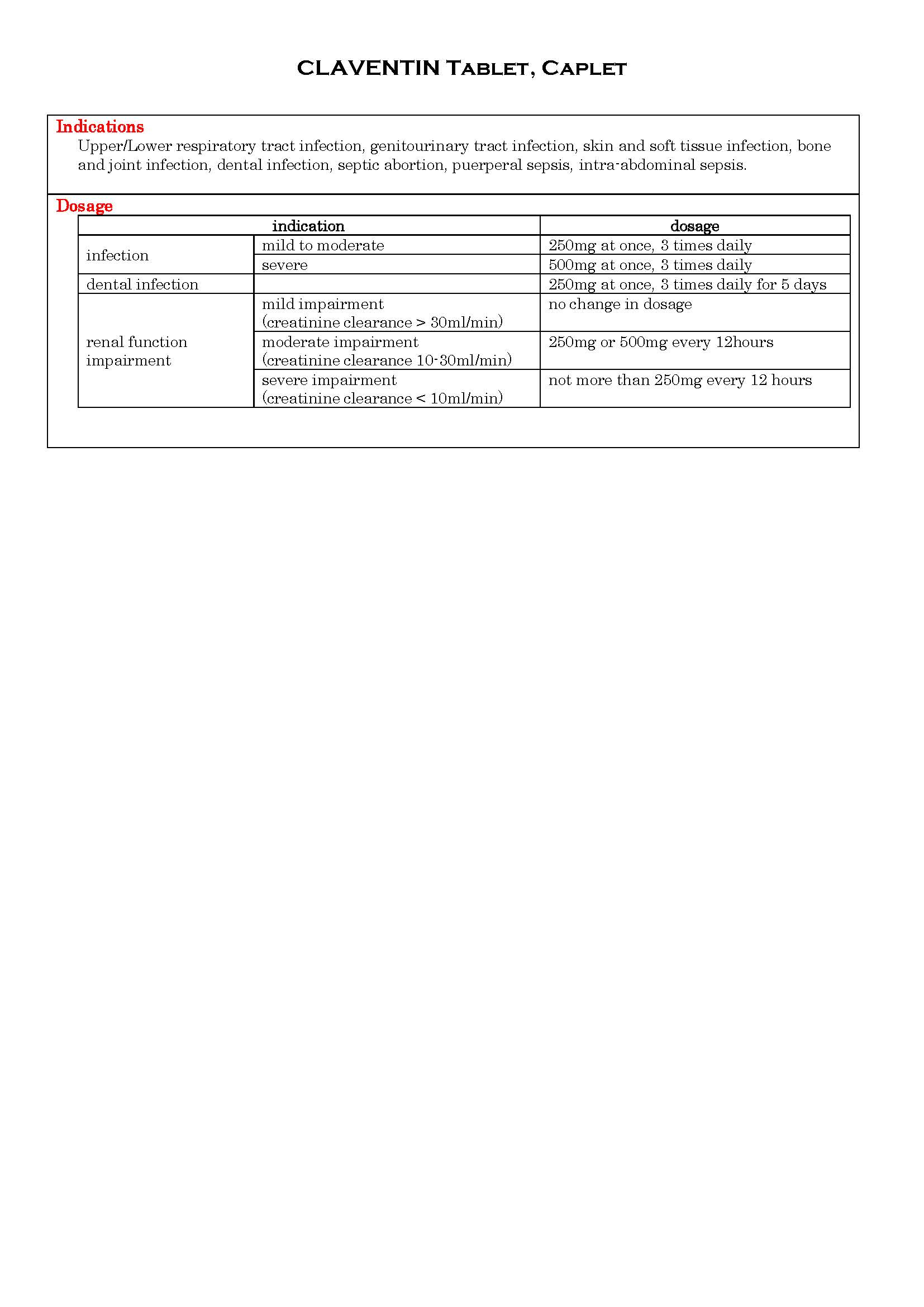
-
ហាមប្រើ
- Hypersensitivity to any penicillin.
- Babies born from mother who is hypersensitive to penicillin
- Patients with evidence of cholestatic jaundice (hepatic dysfunction) which connected with penicillin or Co-amoxiclav treatment.
-
ផលរំខាន
1. Diarrhoea, nausea, vomiting, indigestion, pseudomembranous colitis and candidiasis.
2. A moderate rinse in AST and/or ALT may occur during treatment with semisynthetic penicillins.
3. Hepatitis and cholestatic jaundice may be more severe and continue for several months, especially in adult or elderly patients and slightly more frequently in males. The symptoms may occur during treatment but are more frequently reported after cessation of therapy with a delay of up to 6 weeks. The hepatic events are usually reversible.
4. Urticaria and erythematous rashes.
5. Erythema multiforme, Steven’s-Johnsons syndrome, toxic epidermal necrolysis and exfoliative dermatitis. Treatment should be stopped if these adverse reactions occur.
6. Angioneurotic oedema and anaphylaxis.
7. Interstitial nephritis can occur rarely.
8. Transient leucopenia, thrombocytopenia and anemia hemolytic.
-
អន្តរប្រតិកម្ម
1. Co-amoxiclav may reduce the efficacy of oral contraceptives.
2. Prolongation of bleeding time and prothrombine time in patients receiving anticoagulation therapy.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Administration to pregnancy and lactation women is not recommended, unless considered essential by the physician.
-
ការប្រុងប្រយ័ត្នជាពិសេស
1. Patients with severe hepatic dysfunction.
2. Patients with moderate or severe renal impairment.
3. Administration to pregnancy and lactation women is not recommended, unless considered essential by the physician.
4. 2 tablets CLAVETIN 250mg are not equivalent 1 tablet CLAVENTIN 500mg. Therefore 1 CLAVENTIN 500mg tablet for treatment of more severe infections, should not be substituted with 2 tablets CLAVENTIN 250mg.
5. High dosage and prolonged use may also result super infection) occasionally caused by Enterobacter, Pseudomonas, S.aureus and Candida) in gastrointestinal tract.
-
សកម្មភាពឱសថ
Semi synthetic penicillin derivate antibiotic, it is a broad spectrum, effective against gram positive and gram negative.
Amoxicillin acts by inhibit the mycobacterium cell wall synthesis and characterizes bactericidal.
Clavulanic acid will block betalactamase enzyme which is produced by certain bacteria.
The mechanism of action of clavulanic acid occurs in 2 phases:
1. As competitive inhibitor because the chemical structure of clavulanic acid resembles with penicillin, therefore clavulanic acid can replace the active part of enzyme betalactamase structure without any chemical reaction.
2. Carbonyl betalactamase group from clavulanic acid changes penicillinase enzyme become acyl enzyme. The form of acyl enzyme can not destructive the betalactam ring of penicillin.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
