ZOLOFT Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Pfizer
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
DKSH

- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Sertraline 50mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់


-
ហាមប្រើ
in patients with a known hypersensitivity to sertraline.
Concomitant use in patients taking monoamine oxidase inhibitors (MAOs) , primozide is contraindicated.
-
ផលរំខាន
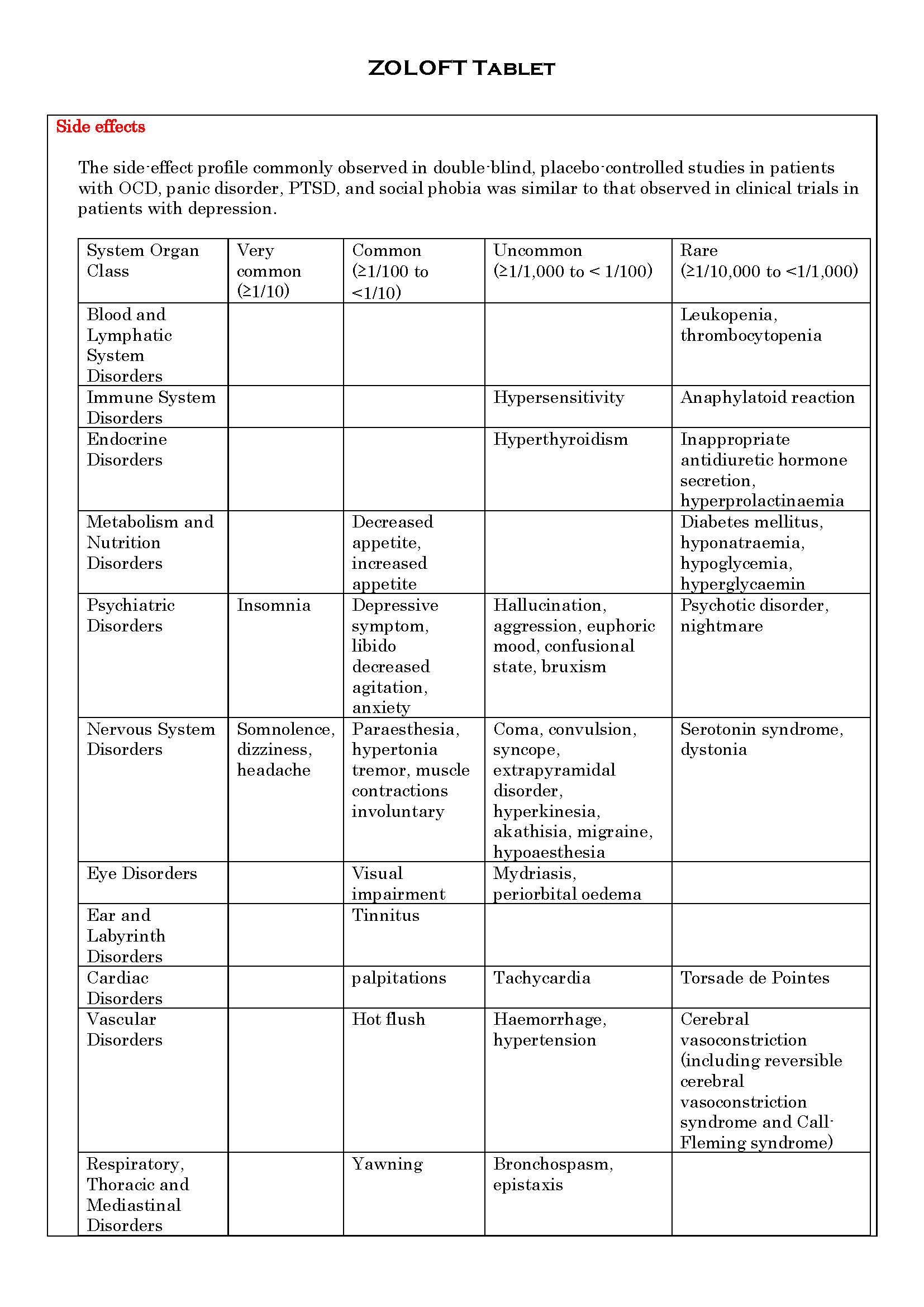
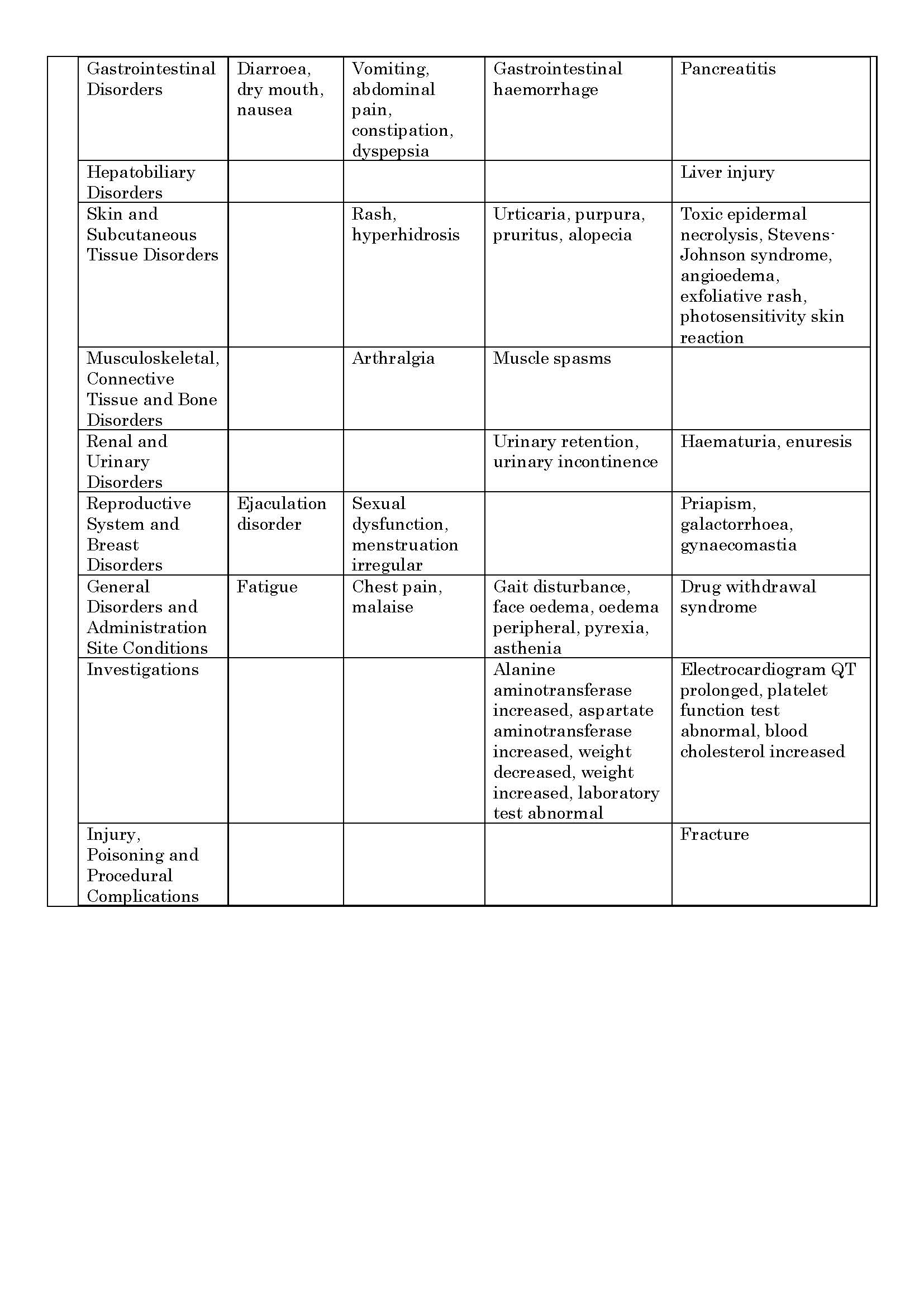
-
អន្តរប្រតិកម្ម
Monoamine Oxidase Inhibitors
Pimozide (increased pimozide levels have been demonstrated in a study of a single low dose pimozide (2mg) with sertraline co-administration. There increased levels were not associated with any changes in EKG. While the mechanism of this interaction is unknown, due to the narrow therapeutic index of pimozide, concomitant administration of sertraline and pimozide is contraindicated.
Drugs that Prolong the QTc Interval
The risk of QTc prolongation and/or ventricular arrhythmias (e.g., TdP) is inreased with concomitant use of other drugs which prolong the QTc interval(e.g., some antipsychotics and antibiotics).
CNS Depressants and Alcohol
The co-administration of sertraline 200mg daily did not potentiate the effects of alcohol, carbamazepine, haloperidol or phenytoin on cognitive and psychomotor performance in healthy subjects; however, the concomitant use of sertraline and alcohol is not recommended.
Lithium
In placebo-controlled trials in normal volunteers, the co-administration of sertraline with lithium did not significantly alter lithium pharmacokinetics, but did result in an increase in tremor relative to placebo, indicating a possible pharmacodynamic interaction. When co-administering sertraline with medications, such as lithium, which may act via serotonergic machanisms, patients should be approriately monitored.
Phenytoin
A placebo-controlled trail in normal volunteers suggests that chronic administration of sertraline 200mg/day does not produce clinically important inhibition of phenytoin metabolism. Nonetheless, it is recommended that plasma phenytoin concentrations be monitored following intiation of sertraline therapy, with appropriate adjustments to the phenytoin dose. In addition,co-administration of phenytoin may cause a reduction of sertraline plasma levels.
Sumatriptan
There have been rare post-marketing reports describing patients with weakness, hyperreflexia, incoordination, confusion, anxiety and agitation following the use of sertraline and sumatriptan, If concomitant treatment with sertraline and sumatriptan is clinically warranted, appropriate observvation of the patient is advised.
Other Serotonergic Drugs (see section "special precaution" entanyl and its analogues, tramadol, dextromethorphan, tapentadol, meperidine, methadone, pentazocine and Serotonin Syndrome).
Protein-bound Drugs
Since sertraline is bound to plasma proteins, the potential of sertraline to interact with other polasma protein-bound drugs should be borne in mind. However, in three formal interaction studies with diazepam, tolbutamie and warfarin, respectively, sertraline was not shown to have significant effects on the protein binding of the substrate.
Warfarin
Co-administration of sertraline 200mg daily with warfarin resulted in a small but satistically significant increase in prothrombin time, the clinical significance of which is unknown. Accordingly, prothrombin time should be carefully monitored when sertraline therapy is initiated or stopped.
Other Drug Interactions
Formal drug interaction stueids have been performed with sertraline. Co-administraion of sertraline 200mg daily with diazepam or tolbutamide resulted in small, statistically significant dhanges in some pharmacokinetic parameters. Co-administration with cimetidine caused a substantial decrease in sertraline clearance. The clinical significance of these changes is unknown. Sertraline had no effect on the beta-adrenergicblocking ability of atenolol. No interaction of sertraline 200mg daily was observed with glibenclamide or digoxin.
Electroconvulsive Therapy (ECT)
There are no clinical studies establising the risks or benefits of the combined use of ECT and sertraline.
Drugs Metabolized by Cytochrome P450 (CYP) 2D6
There is variability among antidepressants in the extent to which they inhibit the activity of isozyme CYP2D6. The clinical significance of this depends on the extent of the inhibition and the therapeutic index of the co-administration drug. CYP2D6 substrates with a narrow therapeutic index include TCAs and class 1C antiarrhythmics shch as propafenone and flecainide. In formalinteraction studies, chronic dosing with sertraline 50mg daily showed minimal elevation (mean 23-37%) of steady-state desipramine plasma levels (a marker of CYP2D6 isoenzyme activity).
Drugs Metabolized by other CYP Enzymes (CYP3A3/4, CYP2C9, CYP2C19, CYP1A2)
-CYP3A3/4: In vivo interaction studies have demonstrated that chronic administration sertraline 200mg daily does not inhibit the CYP3A3/4 mediated 6-beta hydrozylation of endogenous cortisol or the metabolism of carbamazepine or terfenadine. In addition, the chronic administration of sertraline 50mg daily does not inhibit the CYP3A3/4 mediated metabolism of alprazolam. The data suggest that sertraline is not a clinically relevant inhibitor of CYP3A3/4.
- CYP2C9: The apparernt lack of clinically significant effects of the chronic administration of sertraline 200mg daily on plasma concentrations of tolbutamide, phenytoin and warfarin suggests that sertraline is not a clinically relevant inhibitor or CYP2C9.
- CYP2C19 The apparent lack or clinically significant effects of the chronic administration of sertraline 200mg daily on plasma concentrations of diazepam suggests that sertraline is not a clinically relevant in hibitor of CYP2C19.
- CYP1A2: In vitro studies indicate that sertraline has llittle or no potential to inhibi CYP1A2.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, sertraline should be used during pregnancy only if the perceived benefits outweigh the risks.
Isolated studies in small numbers of nursing mothers and their infants indicated negligible or undetectable levels of sertraline in infant serum, although levels in breast milk were more concentrated than in maternal serum. Use in nursing mothers is not recommended unless, in the judgment of the physician, the benefits outweigh the risks.
If sertraline is used during pregnancy and/or lactation, the physician should be aware that symptoms, including those compatible with withdrawal reactions, have been reported in some neonates whose mothers had been on SSRI antidepressants, including sertraline.
Women of childbearing potential should employ an adequate method of contraception if taking sertraline.
-
ការប្រុងប្រយ័ត្នជាពិសេស
- Serotonin Syndrome (SS)
- Monoamine Oxidase Inhibitors
- Other Serotonergic Drugs
- QTc Prolongation/Torsade de Pointes (TdP)
- Switching from Selective Serotonin Reuptake Inhibitors (SSRIs), Antidepressants or Antiobsessional Drugs
- Activation of Mania/Hypomania
- Seizures
- Suicide/Suicidal Thoughts or Clinical Worsening
- Abnormal Bleeding/Haemorrhage
- Hyponatremia
- Bone Fractures
- Use in Hepatic Insufficiency
- Use in Renal Insufficiency
- Diabetes/Loss of Glycemic Control
- Laboratory Tests
- Angle Closure Glaucoma
- Use in Children and Adolescents
-
សកម្មភាពឱសថ
Sertraline is a potent and selective inhibitor of neuronal serotonin (5-HT) reuptake in vitro, which results in the potentiation of the effects of 5-HT in animals. It has only very weak effects on norepinephrine and dopamine neuronal reuptake. At clinical doses, sertraline blocks the uptake of serotonin into human platelets. It is devoid of stimulant, sedative or anticholinergic activity or cardiotoxicity in animals. In controlled studies in normal volunteers, sertraline did not cause sedation and did not interfere with psychomotor performance. In accord with its selective inhibition of 5-HT uptake, sertraline does not enhance catecholaminergic activity. Sertraline has no affinity for muscarinic (cholinergic), serotonergic, dopaminergic, adrenergic, histaminergic, GABA or benzodiazepine receptors. The chronic administration of sertraline in animals was associated with down-regulation of brain norepinephrine receptors as observed with other clinically effective antidepressants and antiobsessional drugs.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
