YASMIN Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Bayer Weimar GmbH und Co. KG, Germany
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
DKSH

- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Ethinylestradiol 0.03mg, Drospirenone 3mg
-
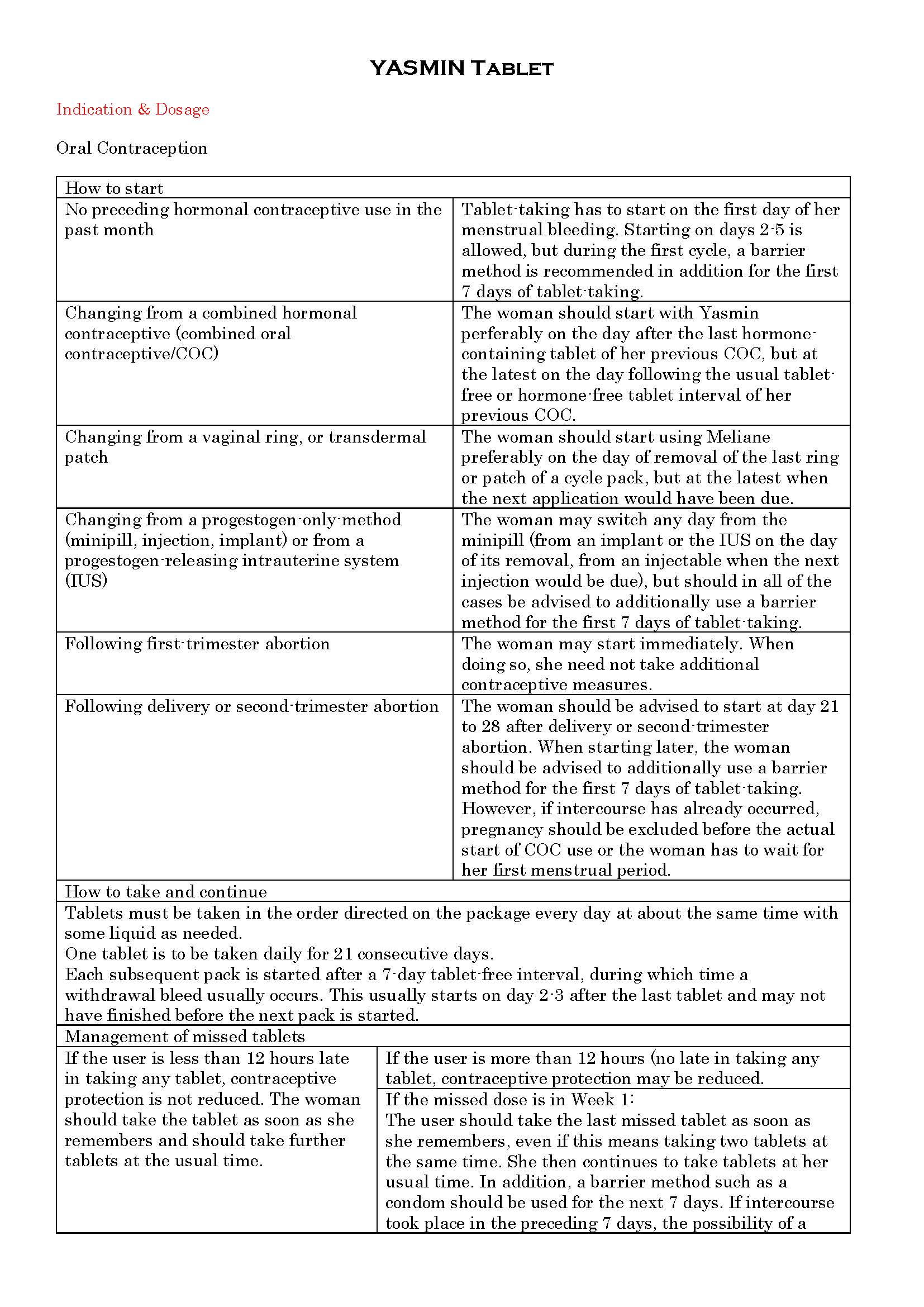
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់

-
ហាមប្រើ
Presence or a history of venous or arterial thrombotic/thromboembolic events (e.g. deep venous thrombosis, pulmonary embolism, myocardial infarction) or of a cerebrovascular accident.
- Presence or history of prodromi of a thrombosis (e.g. transient ischaemic attack, angina pectoris)
- A high risk of venous or arterial thrombosis
- History of migraine with focal neurological symptoms
- Diabetes melitus with vascular symptoms
- Severe hypertension
- Severe dyslipoproteinaemia
- Severe hepatic disease
- Renal impairment
- Presence or history of liver tumours (benign or malignant)
- Known or suspected sex-steroid influenced malignancies (e.g. of the genital organs or the breasts)
- Undiagnosed vaginal bleeding
- Known or suspected pregnancy
- Hypersensitivity to the active substances or to any of the excipients
- Cholestatic jaundice or pregnancy or jaundice with prior pill use
-
ផលរំខាន
Common adverse reactions: nausea, breast pain, depression
Serious adverse reactions:
- Symptoms of pulmonary embolism: Sudden onset of unexplained shortness of breath or rapid breathing; sudden coughing which may bring up blood; sharp chest pain which may increase with deep breathing; sense of anxiety; severe light headedness or dizziness; rapid or irregular heartbeat.
- Symptoms of a cerebrovascular accident: sudden numbness or weakness of the face, arm or leg, especially on one side of the body; sudden confusion, trouble speaking or understanding; sudden trouble seeing in one or both eyes; sudden trouble walking, dizziness, loss of balance or coordination; sudden, severe or prolonged headache with no known cause; loss of conciousness
- Symptoms of vascular occlusion: sudden pain, swelling and slight blue discoloration of an extremity; acute abdomen.
- Symptoms of MI: pain, discomfort, pressure, heaviness, sensation of squeezing or fullness in the chest, arm or below the breastbone; discomfort radiating to the back, jaw, throat, arm, stomach; fullness, indigestion or choking feeling; sweating, nausea, vomiting or dizziness; extreme weakness, anxiety, or shortness of breath; rapid or irregular heartbeats.
- Symptoms of liver tumors: severe upper abdominal pain, liver enlargement or signs of intra-abdominal hemorrhage.
- Crohn’s disease and ulcerative colitis
- Chloasma
-
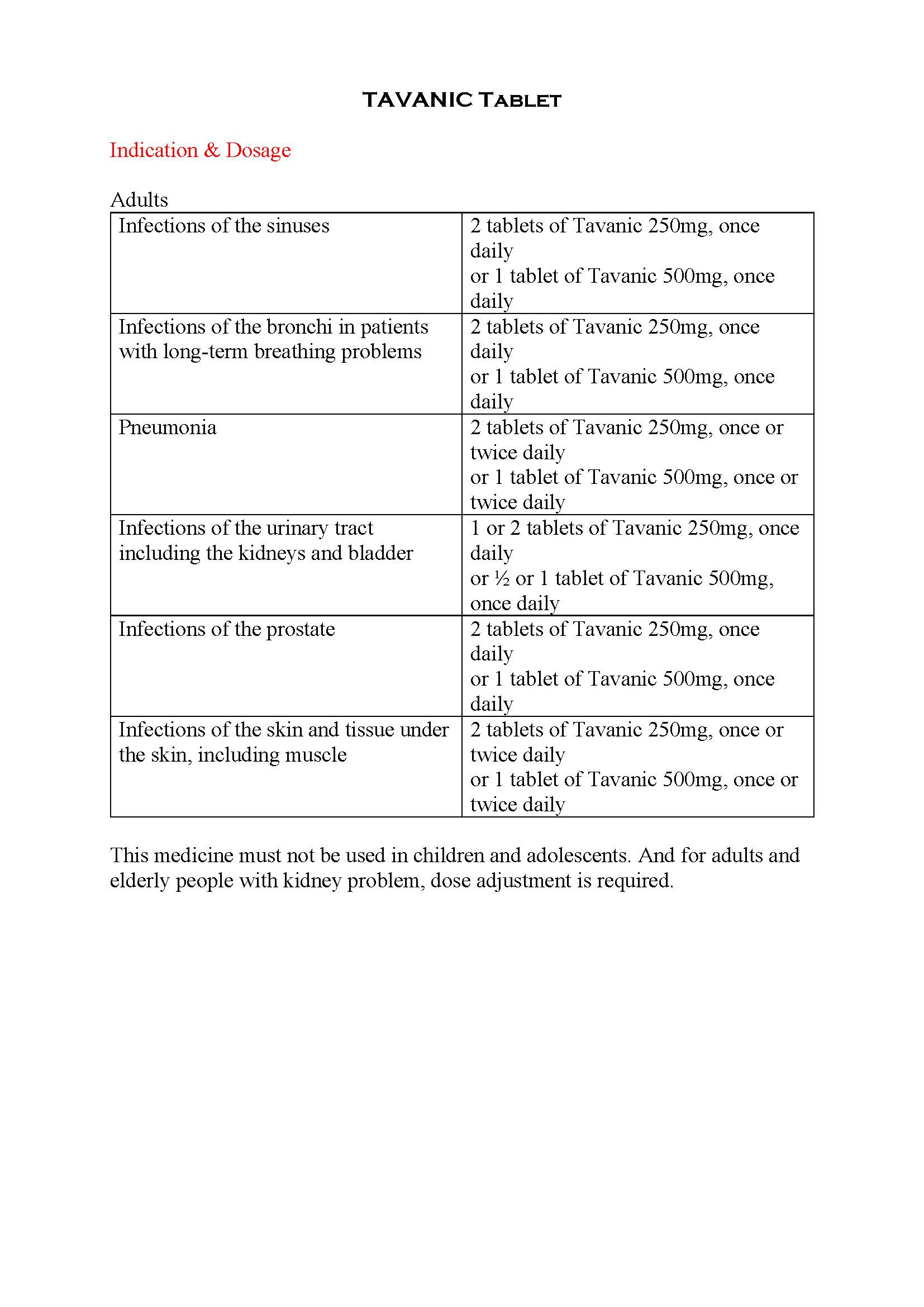
អន្តរប្រតិកម្ម
-
ការប្រុងប្រយ័ត្នជាពិសេស
Warnings
If any of the conditions/risk factors mentioned below is present, the benefits of COC use should be weighed against the possible risks for each individual woman and discussed with the woman before she decides to start using it. In the event of aggravation, exacerbation or first appearance of any of these conditions or risk factors, the woman should contact her physician. The physician should then decide on whether COC use should be discontinued.
Circulatory Disorders
Epidemiological studies have suggested an association between the use of COCs and an increased risk of arterial and venous thrombotic and thromboembolic diseases such as myocardial infarction, deep venous thrombosis, pulmonary embolism and of cerebrovascular accidents. These events occur rarely.
The risk of venous thromboembolism (VTE) is highest during the first year of use. This increased risk is present after initially starting a COC or restarting (following a 4 week or greater pill free interval) the same or a different COC.
Other medical conditions which have been associated with adverse circulatory events include diabetes mellitus, systemic lupus erythematosus, haemolytic uraemic syndrome and chronic inflammatory bowel disease (Crohn’s disease or ulcerative colitis) and sickle cell disease.
An increase in frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation of the COC.
Tumors
The most important risk factor for cervical cancer is persistent HPV infection. Some epidemiological studies have indicated that long-term use of COCs may further contribute to this increased risk but there continues to be controversy about the extent to which this finding is attributable to the confounding effects, e.g., cervical screening and sexual behavior including use of barrier contraceptives.
In rare cases, benign liver tumors, and even more rarely, malignant liver tumors have been reported in users of COCs. In isolated cases, these tumours have led to life-threatening intra-abdominal hemorrhages. A hepatic tumor should be considered in the differential diagnosis when severe upper abdominal pain, liver enlargement or signs of intra-abdominal hemorrhage occur in women taking COCs.
Malignancies may be life-threatening or may have a fatal outcome.
Other conditions
A theoretical risk for hyperkalemia can be assumed only for patients with renal impairment only for patients whose pretreatment serum potassium is in upper reference range, and who are additionally using potassium sparing drugs.
Potassium excretion capacity may be limited in patients with renal insufficiency. Yasmin is contraindicated in real impairment.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
Although small increases in blood pressure have been reported in many women taking COCs, clinically relevant increases are rare.
The following conditions have been reported to occur or deteriorate with both pregnancy and COC use, but the evidence of an association with COC use is inconclusive: jaundice and/or pruritus related to cholestasis; gallstone formation; porphyria; systemic lupus erythematosus; hemolytic uremic syndrome; Sydenham’s chorea; herpes gestationis; otosclerosis-related hearing loss. In women with hereditary angioedema exogenous estrogens may induce or exacerbate symptoms of angioedema.
Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal. Recurrence of cholestatic jaundice which occurred first during pregnancy or previous use of sex steroids necessitates the discontinuation of COCs.
Although COCs may have an effect on peripheral insulin resistance and glucose tolerance, there is no evidence for a need to alter the therapeutic regimen in diabetics using low-dose COCs (containing <0.05mg ethinylestradiol). However, diabetic women should be carefully observed while taking COCs.
Crohn’s disease and ulcerative colitis have been associated with COC use.
Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation whilst taking COCs.
-
សកម្មភាពឱសថ
Inhibition of ovulation and the changes in the cervical secretion.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
