RISEK Capsule
ក្រុមហ៊ុនផលិតឱសថ:
Getz Pharma(Pvt.) Limited, Pakistan.
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
ALLIANCE PHARMA CAMBODGE
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
-
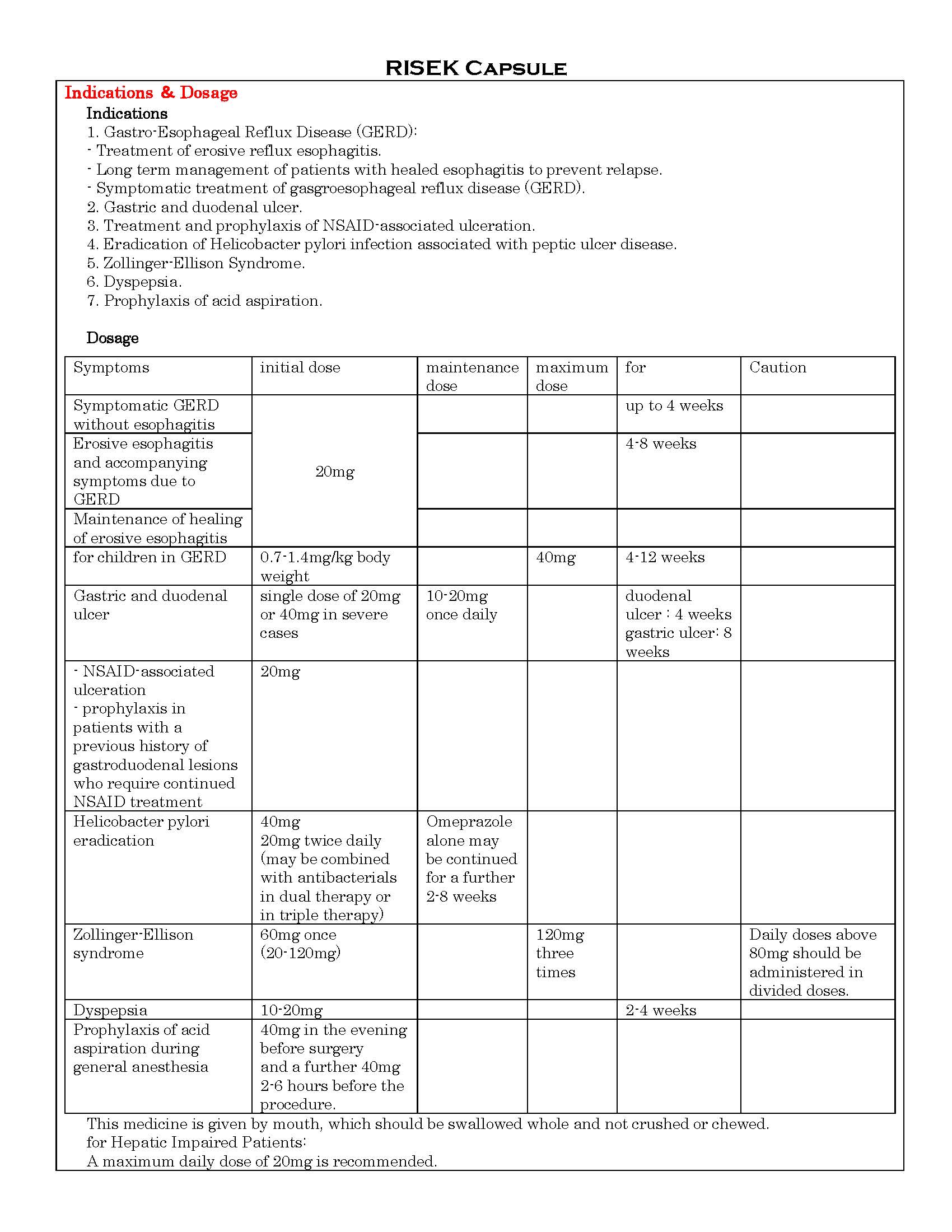
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
-
ហាមប្រើ
in patients with known hypersensitivity to any component of the formulation or to substituted benzimidazoles.
-
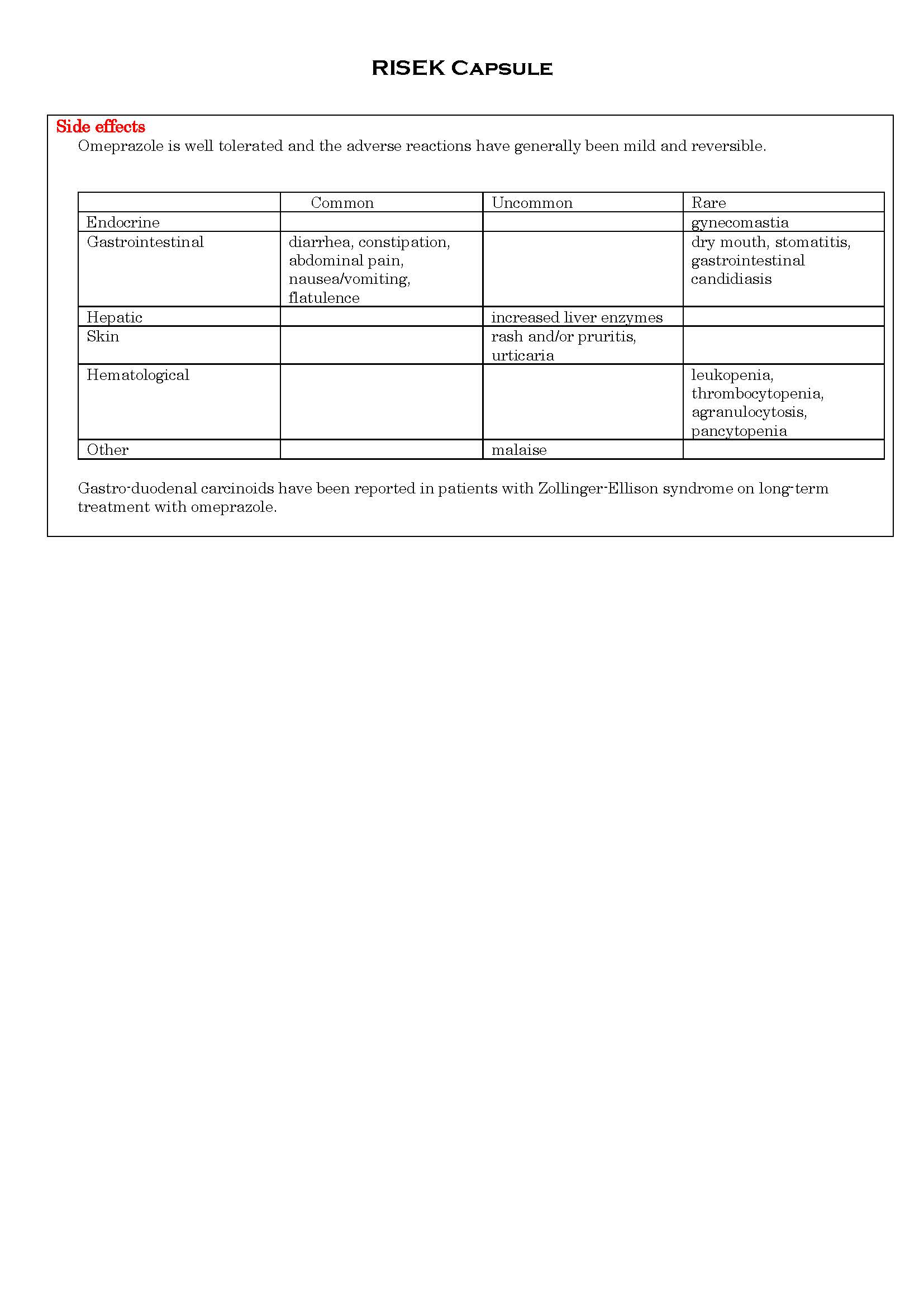
ផលរំខាន
-
អន្តរប្រតិកម្ម
- In common with the use of other inhibitors of acid secretion or antacids, the absorption of ketoconazole, and itraconazole can decrease during treatment with omeprazole due to decreased intragastric acidity during treatment with omeprazole.
- Omeprazole is metabolized by CYP2C19. Thus, when omeprazole is combined with drugs metabolized by CYP2C19, such as diazepam, citalopram, impiramine, clomipramine, phenytoin etc., the plasma concentrations of these drugs may be increased and a dose redction could be needed.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
There are no adequate or well-controlled studies in pregnant women. Omeprazole should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether omeprazole is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from omeprazole, a decision should be made whether, to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ការប្រុងប្រយ័ត្នជាពិសេស
General:
- When gastric ulcer is suspected, the possibility of malignancy should be excluded as treatment may alleviate symptoms and delay diagnosis.
- Prior to initiation of dual or triple therapy, the physician should consider the patient with known hypersensitivity reactions to penicillin, macrolides and other antibiotics.
Hepatic impairment:
Consideration should be given to reducing the dose of omeprazole in patients with impaired hepatic function as bioavailability an half-life can increase.
Pregnancy:
There are no adequate or well-controlled studies in pregnant women. Omeprazole should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers:
It is not known whether omeprazole is excreted in human milk. Because may drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from omeprazole, a decision should be made whether, to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Drug interactions
- In common with the use of other inhibitors of acid secretion or antacids, the absorption of ketoconazole, and itraconazole can decrease during treatment with omeprazole due to decreased intragastric acidity during treatment with omeprazole.
- Omeprazole is metabolized by CYP2C19. Thus, when omeprazole is combined with drugs metabolized by CYP2C19, such as diazepam, citalopram, imipramine, clomipramine, phenytoin etc., the plasma concentrations of these drugs may be increased and a dose reduction could be needed.
-
សកម្មភាពឱសថ
Omeprazole reduces gastric acid secretion through a unique mechanism of action. Omeprazole belongs to a new class of anti-secretory compounds, the substituted benzimidazoles that do not exhibit anti-cholinergic or histamine antagonistic properties. It inhibits secretion of gastric acid by irreversibly blocking the enzyme system of hydrogen/potassium adenosine triphosphatase(H+/K+ ATPase), the proton pump of the gastric parietal cell. This effect is dose-related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
