PROMTO Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Getz Pharma(Pvt.) Limited, Pakistan.
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
ALLIANCE PHARMA CAMBODGE

- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
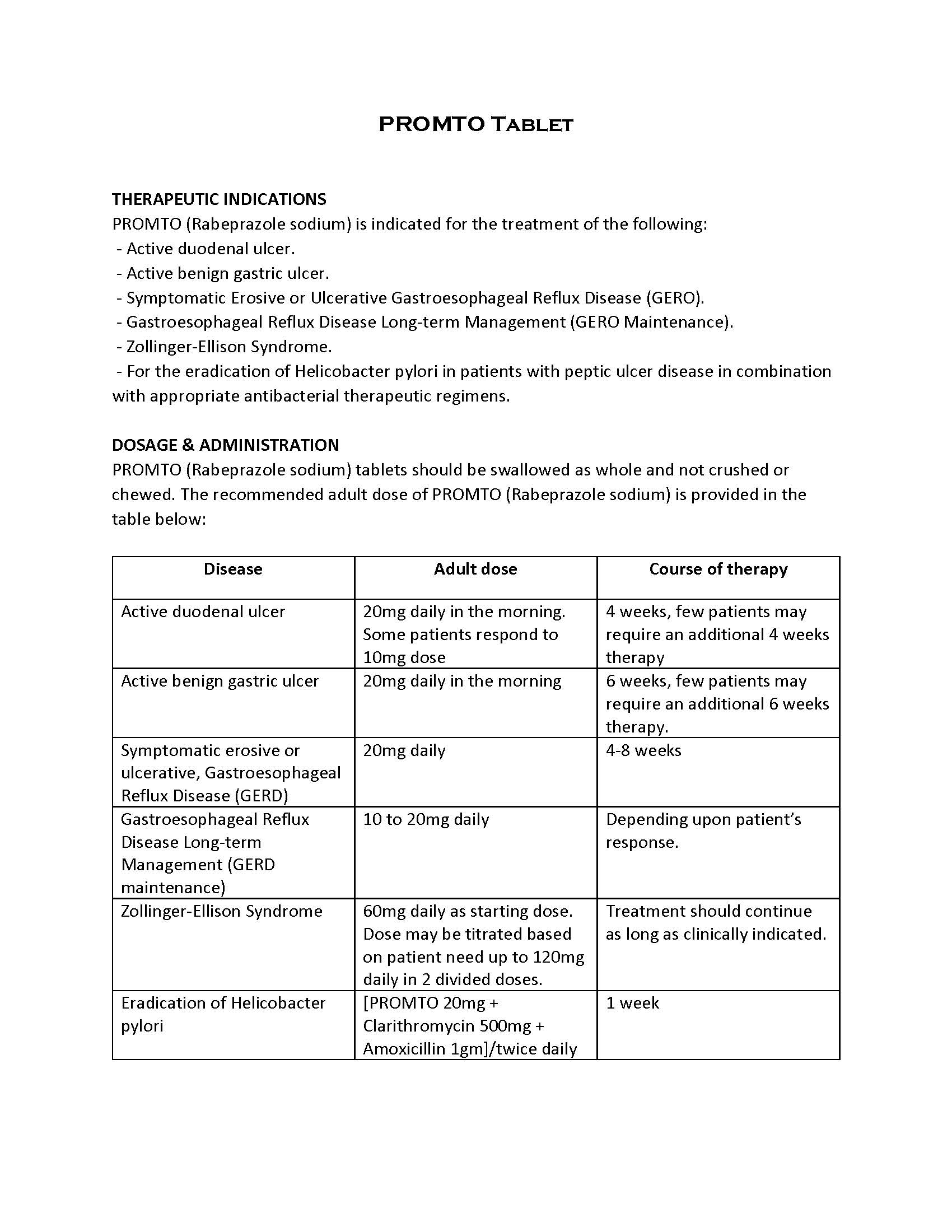
-
ហាមប្រើ
Rabeprazole sodium is contraindicated:
- In patients with known hypersensitivity to rabeprazole or other substituted benzimidazoles or any component of this product.
- In pregnancy and during breast-feeding.
Rabeprazole sodium is not recommended for children, as there is no experience of its use in this group.
-
ផលរំខាន
Rabeprazole sodium Is usually very well tolerated. However, following are the adverse events reported during therapy with rabeprazole sodium.
Common: Headache, diarrhoea and nausea. Other adverse events were rhinitis, abdominal pain, asthenia, flatulence, pharyngitis, vomiting, non-specific pain/back pain, dizziness, flu-like syndrome, Infection, cough, constipation and insomnia.
Less frequent: Rash, myalgia, chest pain, dry mouth, dyspepsia, nervousness, somnolence, bronchitis, sinusitis, chills, eructation, leg cramps, urinary tract infection, arthralgia and fever.
Rare: Anorexia, gastritis, weight gain, depression, pruritus, vision or taste disturbances, stomatitis, sweating and leucocytosis, thrombocytopenia, neutropenia and leukopenia.
-
អន្តរប្រតិកម្ម
Drug Interactions:
Rabeprazole sodium produces a profound and long-lasting inhibition of gastric acid secretion. An interaction with compounds whose absorption is pH dependent may occur. Co-administration of rabeprazole sodium with ketoconazole or itraconazole may result in a significant decrease in antifungal plasma levels. Therefore, individual patients may need to be monitored to determine if a dosage adjustment is necessary when ketoconazole or itraconazole are taken concomitantly with rabeprazole.
-
ការប្រុងប្រយ័ត្នជាពិសេស
- The possibility of malignancy should be excluded prior to commencing treatment with rabeprazole as symptomatic response to therapy with rabeprazole sodium does not preclude the presence of gastric or esophageal malignancy. Patients on long-term treatment (particularly those treated for more than a year) should be kept under regular surveillance.
- Rabeprazole sodium should be used with caution in patients with severe hepatic dysfunction.
-
សកម្មភាពឱសថ
Rabeprazole sodium belongs to a new class of antisecretory compounds (substituted benzimidazole proton-pump inhibitor) that do not exhibit anticholinergic or histamine H2-receptor antagonist properties, but suppress gastric acid secretion by inhibiting the gastric H+/K+ ATPase at the parietal cell. The effect is dose related and leads to the inhibition of both basal and stimulated acid secretion irrespective to the stimulus.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
