PARIET Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Bushu Pharmaceuticals Ltd. Misato factory, japan
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
DKSH
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
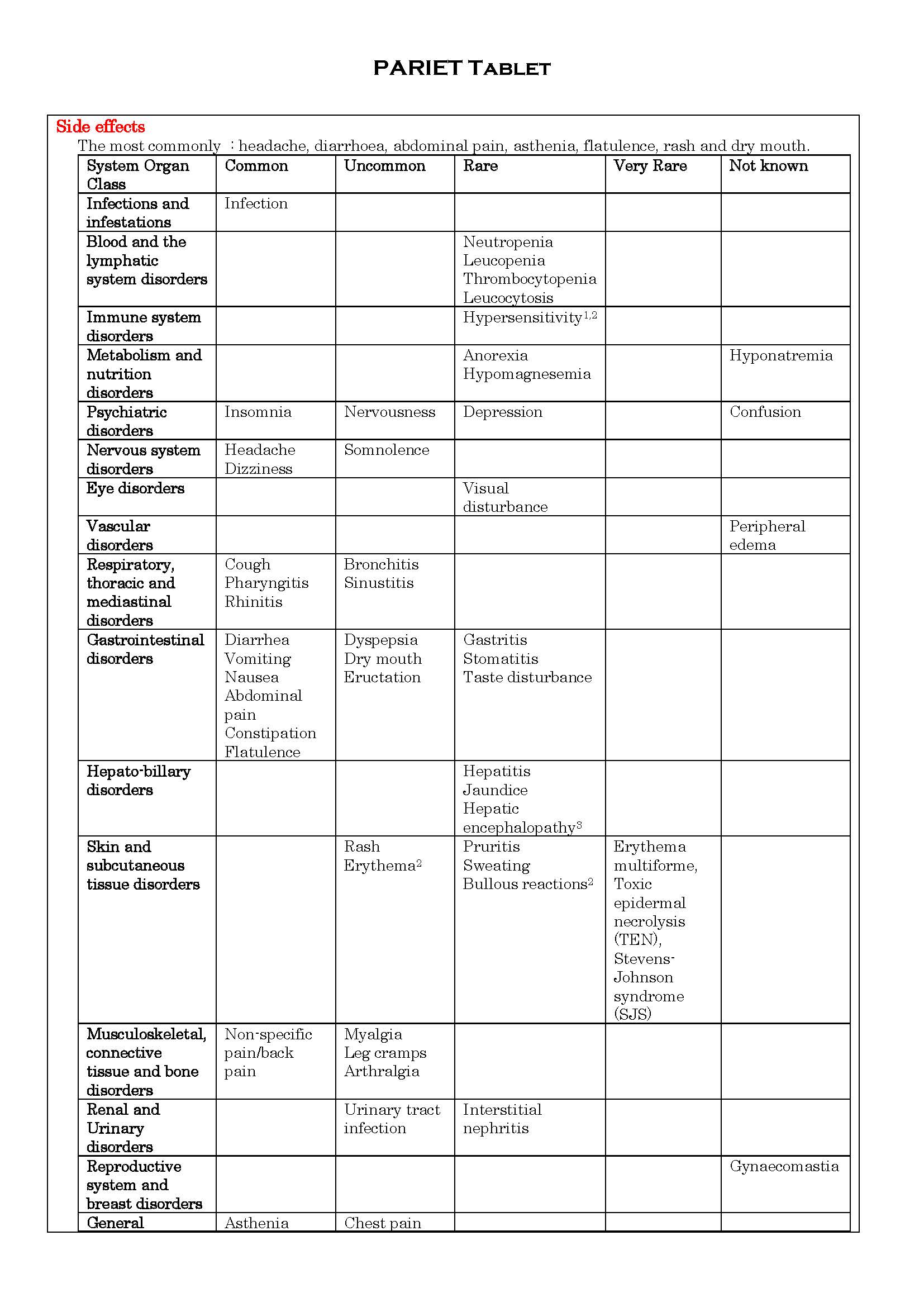
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
គុណភាពព្យាបាល
- ជម្ងឺដំបៅគល់ពោះវៀនតូច
- ជម្ងឺដំបៅក្រពះធម្មតា
- Anastomatic Ulcer
- ជម្ងឺរលាកឬដំបៅក្រពះបណ្ដាលពីការច្រាលជាតិអាស៊ីតឬអាហារចេញពីក្រពះតាមបំពង់អាហារ
- Gastro-Esophageal Reflux Disease Long-term Management
- ជម្ងឺច្រាលជាតិអាស៊ិតឬអាហារចេញពីក្រពះតាមបំពង់អាហារធ្ងន់ធ្ងរ
- Zollinger-Ellison syndrome
- ប្រើរួមជាមួយអង់ទីប៊ីយូទីកចំពោះអ្នកជម្ងឺដំបៅក្រពះក្នុងការសំលាប់មេរោគ Helicobacter pylori។
កំរិត និងរបៀបប្រើ
មនុស្សពេញវ័យ និងមនុស្សចាស់៖
- ជម្ងឺដំបៅគល់ពោះវៀនតូច ជម្ងឺដំបៅក្រពះធម្មតា Anastomatic Ulcer: 10mg ឬ 20mg លេបតែម្ដងក្នុង1ថ្ងៃ នៅពេលព្រឹក
- ជម្ងឺរលាកឬដំបៅក្រពះបណ្ដាលពីការច្រាលជាតិអាស៊ីតឬអាហារចេញពីក្រពះតាមបំពង់អាហារ: 10mg ឬ 20mg លេបតែម្ដងក្នុង1ថ្ងៃ សំរាប់រយៈពេល 4 ទៅ 8 អាទិត្យ។
- Gastro-Esophageal Reflux Disease Long-term Management: 10mg ឬ 20mg លេបតែម្ដងក្នុង1ថ្ងៃ រយៈពេលប្រើប្រែប្រួលតាមសភាពអ្នកជម្ងឺ។
- ជម្ងឺច្រាលជាតិអាស៊ិតឬអាហារចេញពីក្រពះតាមបំពង់អាហារធ្ងន់ធ្ងរ: 10mg ប្រើតែម្ដងក្នុង1ថ្ងៃ ចំពោះអ្នកជម្ងឺមិនរលាកបំពង់អាហារ ក្រោយរយៈពេល4អាទិត្យ ប្រសិនបើជម្ងឺមិនបានធូរស្បើយ ត្រូវពិគ្រោះជាមួយគ្រូពេទ្យ។
- Zollinger-Ellison syndrome: កំរិតប្រើប្រែប្រួលតាមសភាពអ្នកជម្ងឺ។ កំរិតដំបូងផ្ដើមពី 60mg ក្នុង1ថ្ងៃ និងកើនឡើងរហូតដល់ 100mgក្នុង1ថ្ងៃ ឬ 60mg 2ដងក្នុង1ថ្ងៃ រយៈពេលប្រើរហូតដល់បាត់រោគសញ្ញា។
- ការបំបាត់ជម្ងឺក្រពះដោយ H. pylori : ត្រូវព្យាបាលរយៈពេល7ថ្ងៃIndications&Dosage
- Active Duodenal Ulcer
- Active Benign Gastric Ulcer
- Anastomotic Ulcer
- Erosive or Ulcerative Gastro-Esophageal Reflux Disease (GERD).
- Gastro-Esophageal Reflux Disease Long-term Management (GERD Maintenance)
- Symptomatic Treatment of Moderate to Very Severe Gastro-Esophageal Reflux Disease (symptomatic GERD)
- Zollinger-Ellison Syndrome and other pathological hypersecretory conditions
- In combination with appropriate antibacterial therapeutic regimens for the eradication of Helicobacter pylori in patients with peptic ulcer disease.
Dosage
Adults/elderly:
Active Duodenal Ulcer, Active Benign Gastric Ulcer and Anastomotic Ulcer:
The recommended oral dose is 10mg or 20mg to be taken once daily in the morning.
Most patients heal within 4 weeks. However a few patients may require an additional 4weeks of therapy to achieve healing. Most patients with active benign gastric ulcer heal within 6 weeks. However again a few patients may require an additional 6 weeks of therapy to achieve healing.
Erosive or Ulcerative Gastro-Esophageal Reflux Disease (GERD):
The recommended oral dose for this condition is 10mg or 20mg to be taken once daily for four to eight weeks.
Doses of 10mg or 20mg twice daily may be administered orally to reflux esophagitis patients for a further 8 weeks when PPI treatment is ineffective. However, a dose of 20mg twice daily should only be administered to patients with severe mucosa injury.
GERD Maintenance:
For long-term management a maintenance dose of PARIET® 10mg or 20mg once daily can be used depending upon patient response.
Symptomatic GERD:
10mg once daily in patients without esophagitis. If symptom control has not been achieved during 4 weeks, the patient should be further investigated. Once symptoms have resolved, subsequent symptom control can be achieved using an on-demand regimen taking 10mg once daily when needed.
Zollinger-Ellison Syndrome and other pathological hypersecretory conditions:
The dose necessary varies with the individual patient. A starting dose of 60mg daily, and doses of up to 100mg once daily, or 60mg twice daily have been used. Some patients may require divided doses. Dosing should continue for as long as clinically necessary. Some patients have been treated continuously for up to 1 year.
Eradication of H. pylori:
Patients with H. pylori infection should be treated with eradication therapy. The following combination given for 7 days is recommended.
PARIET® 20mg twice daily + clarithromycin 500mg twice daily and amoxicillin 1g twice daily.
For indications requiring once daily treatment PARIET® tablets should be taken in the morning, before eating; and although neither the time of day nor food intake was shown to have any effect on rabeprazole sodium activity, this regimen will facilitate treatment compliance.
Patients should be cautioned that the PARIET® tablets should not be chewed or crushed, but should be swallowed whole.
Renal and hepatic impairment:
No dosage adjustment is necessary for patients with renal or hepatic impairment.
Children:
Safety and effectiveness of rabeprazole 20mg for the short-term (up to 8weeks) treatment of GERD in adolescents 12 years of age and above is supported by a) extrapolation of results from adequate and well-controlled studies that supported the effectiveness of rabeprazole sodium for adults; b) safety and pharmacokinetic studies performed in adolescent patients. The recommended oral dose for adolescents 12 years of age and above is 20mg once daily for up to 8 weeks.
The safety and effectiveness of rabeprazole sodium for the treatment of GERD in children <12 years of age have not been established. The safety and effectiveness of rabeprazole sodium for other uses have not been established in pediatric patients.
-
ហាមប្រើ
ហាមប្រើចំពោះអ្នកជម្ងឺងាយមានប្រតិកម្មជាមួយសារធាតុផ្សំណាមួយនៃឱសថនេះ។
in patients with known hypersensitivity to rabeprazole sodium, substituted benzimidazoles or to any excipient used in the formulation.
-
ផលរំខាន

-
អន្តរប្រតិកម្ម
ការប្រើឱសថនេះរួមជាមួយ Ketoconazole ឬ Itraconazole ធ្វើអោយថយចុះយ៉ាងធ្ងន់ធ្ងរនូវកំរិតរបស់ Antifungal ក្នុងប្លាស្មា។
Rabeprazole sodium produces a profound and long lasting inhibition of gastric acid secretion. An interaction with compounds whose absorption is pH dependent may occur. Co-administration of rabeprazole sodium with ketoconazole or itraconazole may result in a significant decrease in antifungal plasma levels. Therefore individual patients may need to be monitored to determine if a dosage adjustment is necessary when ketoconazole or itraconazole are taken concomitantly with PARIET.
In clinical trials, antacids were used concomitantly with the administration of PARIET® and, in a specific drug-drug interaction study, no interaction with liquid antacids was observed.
Co-administration of atazanavir 300 mg/ritonavir 100 mg with omeprazole (40 mg once daily) or atazanavir 400 mg with lansoprazole (60 mg once daily) to healthy volunteers resulted in a substantial reduction in atazanavir exposure. The absorption of atazanavir is pH dependent. Although co-administration with rabeprazole was not studied, similar results are expected with other proton pump inhibitors. Therefore PPIs, including rabeprazole, should not be coadministered with atazanavir.
Case reports, published population pharmacokinetic studies, and retrospective analyses suggest that concomitant administration of PPIs and methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxy methotrexate. However, no formal drug interaction studies of methotrexate with PPIs have been conducted.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy:
There are no data on the safety of rabeprazole in human pregnancy. Reproduction studies performed in rats and rabbits have revealed no evidence of impaired fertility or harm to the fetus due to rabeprazole sodium, although low feto-placental transfer occurs in rats.
Lactation:
It is not known whether rabeprazole sodium is excreted in human breast milk. No studies in lactating women have been performed. Rabeprazole sodium is however excreted in rat mammary secretions. Therefore PARIET® should not be used during breast feeding.
-
ការប្រុងប្រយ័ត្នជាពិសេស
ការប្រុងប្រយ័ត្ន៖
- ត្រូវមានការត្រួតពិនិត្យជាប្រចាំ និងអោយបានទៀងទាត់ក្នុងការព្យាបាលដោយឱសថនេះរយៈពេលយូរ (លើសពី1ឆ្នាំ)
- ត្រូវលេបឱសថនេះទាំងមូល នៅពេលព្រឹកមុនស្រស់ស្រូបអាហារ។
- អ្នកបើកបរយានយន្ត និងបញ្ជាគ្រឿងម៉ាស៊ីន គួរជៀសវាងប្រើ (បណ្ដាលអោយងងុយដេក)
- ឱសថនេះមិនត្រូវប្រើចំពោះកូនក្មេងឡើយSymptomatic response to therapy with rabeprazole sodium does not preclude the presence of gastric or esophageal malignancy, therefore the possibility of malignancy should be excluded prior to commencing treatment with PARIET®.
Patients on long-term treatment (particularly those treated for more than a year) should be kept under regular surveillance.
Patients should be cautioned that PARIET® tablets should not be chewed or crushed, but should be swallowed whole.
PARIET® is not recommended for use in children <12 years of age, as there is no experience of its use in this group.
No evidence of significant drug related safety problems was seen in a study of patients with mild to moderate hepatic impairment versus normal age and sex matched controls. However because there are no clinical data on the use of PARIET® in the treatment of patients with severe hepatic dysfunction the prescriber is advised to exercise caution when treatment with PARIET® is first initiated in such patients.
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically.
Observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, and long-term PPI therapy (a year or longer).
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients.
Treatment with PPI may possibly increase the risk of gastrointestinal infections such as Clostridium difficile.
-
សកម្មភាពឱសថ
Rabeprazole sodium belongs to the class of anti-secretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or H2 histamine antagonist properties, but suppress gastric acid secretion by the specific inhibition of the H+/K+-ATPase enzyme (the acid or proton pump). The effect is dose-related and leads in inhibition of both basal and stimulated acid secretion irrespective of the stimulus. Animal studies indicated that after administration, rabeprazole sodium rapidly disappears from both the plasma and gastric mucosa. As a week base, rabeprazole is rapidly absorbed following all doses and is concentrated in the acid environment of the parietal cells. Rabeprazole is converted to the active sulphenamide from through protonation and it subsequently reacts with the available cysteines on the proton pump.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
