GARBAPIA Capsule
ក្រុមហ៊ុនផលិតឱសថ:
DAEWOONG PARMACEUTICAL CO., LTD., Korea
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Gabapentin 300mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
1. Epilepsy
- Single therapy (including the treatment for a patient who is diagnosed as a seizure newly): Simple/complex partial seizure with and without secondary generalization in patients over 13 years of age.
-Adjunctive therapy: Partial seizures with and without secondary generalization in children over 3 years of age or adults.
2. Neuropathic pain
Dosage & Administration
1. Epilepsy
1) Single and adjunctive therapy in patients over 13 years of age
Phased titration to make a maintenance dose 900mg/day can be accomplished for the first 3 days as following:
(1) Day 1: 300mg once a day.
(2) Day 2: 3000mg twice a day.
(3) From Day3: 300mg three times a day.
Other than the above method, 300mg capsule 3 times a day may be administered as a starting dose.
If necessary, dosage can be increased to 1200mg/day of gabapentin, daily total dosage should not exceed 2400mg. (There is no sufficient safety and efficacy data regarding the overdosage of 2400mg/day of gabapentin.)
2) Adjunctive therapy in patients 3-12 years of age
Phased titration to make a maintenance dose 30mg/weight (kg) can be accomplished for the first 3 days as followings.
(1) Day 1: Gabapentin 10mg/kg/day
(2) Day 2: Gabapentin 20mg/kg/day
(3) From Day 3: Gabapentin 30mg/kg/day
Dosage may be increased to 40-50mg/kg/day, if necessary. Total daily dose should be given in 3 equally divided doses.
2. Neuropathic pain
1) Adults (Over the age of 18): Phased titration to make a maintenance dose 900mg/day can be accomplished for the first 3 days as followings.
(1) Day 1: 300mg once a day.
(2) Day 2: 300mg twice a day.
(3) From Day 3: 300mg 3 times a day.
Other than the above method, 300mg 3 times a day may be administered as a starting dose. (900mg/day) daily dose should be given in 3 equally divided doses.
3. Children:
When this medicine is used as adjunctive therapy in children 3-12 years of age, 300mg capsules may be administered instead of high-dosage of gabapentin tab.
(See the package insert about the details below.)
4. Invalid:
5. Elderly:
6. Patients with renal impairment or undergoing hemodialysis (creatinine clearance <80mL/min)
7. Administration method and period
- This capsule should be swallowed with sufficient liquid, and it is given orally with or without food. Given in 3 divided doses, the maximum time interval between doses should not exceed 12 hours.
- If the administration of this drug is skipped (in case that 12 hrs have been passed since the last administration), a doctor has to make a decision on additional administration.
- When GARBAPIA and antacids containing aluminum and magnesium are used concomitantly, it is recommended that GARBAPIA is taken about 2 hours following any such antacid administration. It will help to deceased the reduction rate of bioavailability.
- Administration period of this drug should be different from each clinical demand. For the treatment of epilepsy, generally long-term administration is needed. Rebound phenomenon (seizure frequencies are increased because of gabapentin) doesn’t occur. In case of discontinuation of this drug or concurrent use of this drug with other anticonvulsants, it should be changed gradually at least for 1 week.
- Safety and efficacy has been evaluated over 5 months for the treatment of neuropathic pain.
- To reduce main adverse events such as somnolence, vertigo, fatigue and ataxia, first administration at a first day should be performed while sleeping.
-
ហាមប្រើ
- Patients who have demonstrated hypersensitivity to this drug or its ingredients.
- Patients who acute pancreatitis.
- Patients with generalized absence seizure.
- Patients with galactosaemia (galactose intolerance). This drug contains galactose ingredient.
-
ផលរំខាន
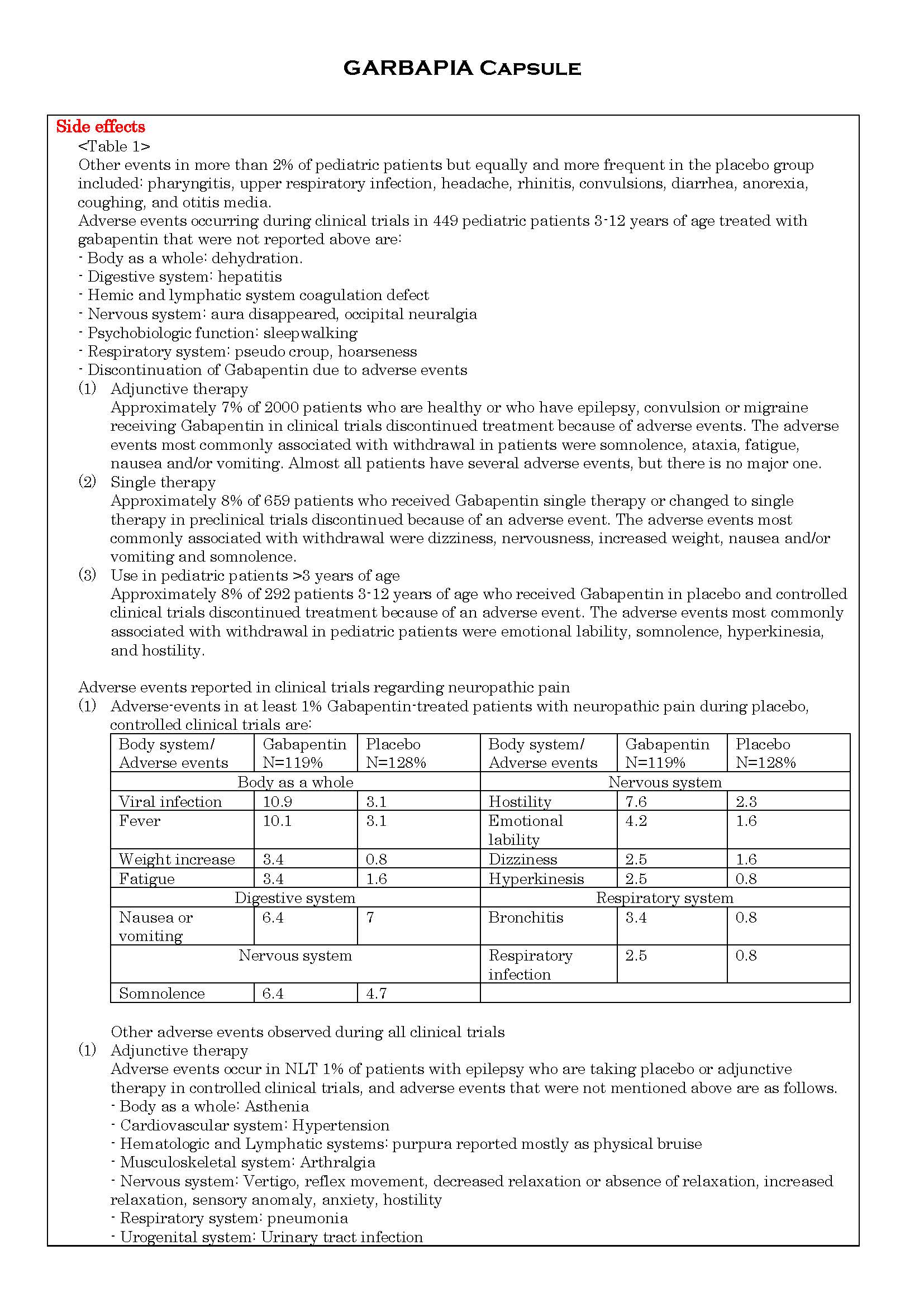
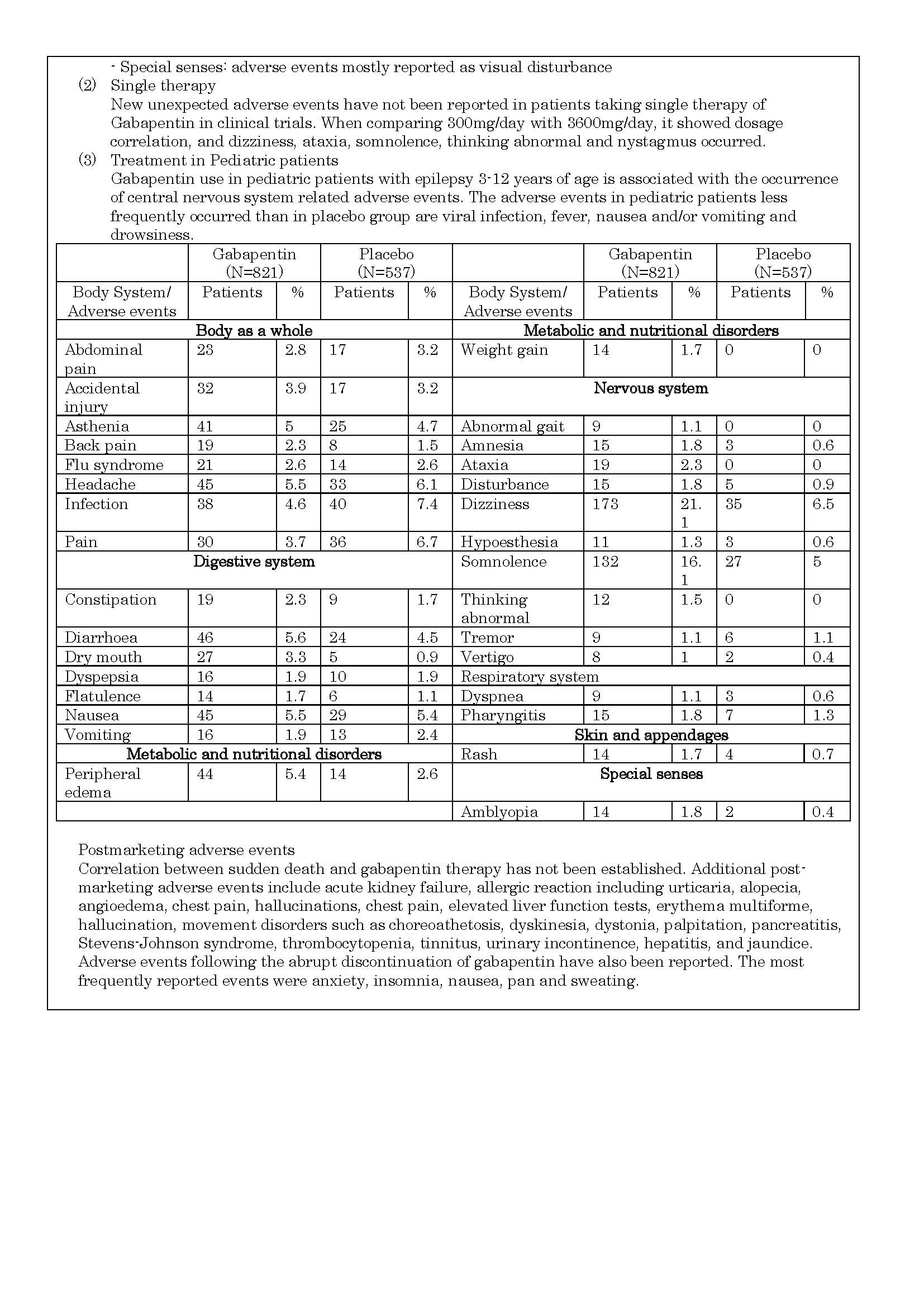
-
អន្តរប្រតិកម្ម
(See the package insert about the details below.)
- Morphine
- There is no interaction between Gabapentin and Phenobarbital, Phenytoin, Valproic Acid or Carbamazepine. Gabapentin steady-state pharmacokinetics are similar for healthy subjects and patients with epilepsy receiving anti-epileptic agents.
- Oral contraceptives including norethisterone and/or ethinyl oestradiol
- An aluminum and magnesium containing antacid
- Renal excretion of Gabapentin is unaltered by probenecid.
- Cimetidine
- Abuse of alcohol or CNS drugs
- Naproxen
- Hydrocodone
- Drug/Laboratory Test Interactions
Because false positive readings were reported with the Ames N-Multistix SG dipstick test for urinary protein when gabapentin was added to other antiepileptic drugs, the more specific sulfosalicylic acid precipitation procedure is recommended to determine the presence of urine protein.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
There are no adequate and well-controlled studies in pregnant women. Animal reproduction studies are no always predictive of human response. This drug should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Lactation
Gabapentin is excreted in human milk but the effect on the nursing infant is unknown. Gabapentin should be used in women who are nursing only if the benefits clearly outweigh the risks.
-
ការប្រុងប្រយ័ត្នជាពិសេស
- Although there is no evidence of rebound seizures with Gabapentin, abrupt withdrawal of anticonvulsant agents in epileptic patients may precipitate status epileptics.
-patients who require concomitant treatment with morphine may experience increases in Gabapentin concentrations. patients should be carefully observed for signs of CNS depression, such as somnolence, and the dose of this drug or morphine should be reduced appropriately.
- patients with renal impairment should take reduced dose of this drug.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
