DUPHASTON Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Abbott Biologicals B.V., Netherlands
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Dydrogesterone 10mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
Indications:
- Symptomatic relief of mild to moderate vasomotor symptoms associated with menopause.
- Prevention of postmenopausal osteoporosis, if other treatments are unsuitable.
Progesterone deficiencies:
- Treatment of dysmenorrhoea.
- Treatment of endometriosis.
- Treatment of secondary amenorrhoea.
- Treatment of irregular cycles.
- Treatment of dysfunction uterine bleeding.
- Treatment of threatened abortion.
- Treatment of habitual abortion.
- Treatment of infertility due to luteal insufficiency.
Dosage:
Dosage, treatment schedule and duration of treatment may be adapted to the severity of the dysfunction and the clinical response.
Symptomatic relief of mild to moderate vasomotor symptoms associated with menopause, prevention of postmenopausal osteoporosis,
- Continuous sequential therapy: An oestrogen is dosed continuously and one tablet of mg dydrogesterone is added for the last 14 days of every 28 day cycle, in a sequential manner.
- Cyclic therapy: When an oestrogen is dosed cyclically with a treatment-free interval, usually 21 days on and 7 days off. One tablet of 10 mg dydrogesterone is added for the last 12-14 days of oestrogen therapy.
If ultrasound or endometrial biopsies would reveal inadequate progestational response, 20 mg dydrogesterone should be prescribed.
Dysmenorrhoea:
10 mg twice daily from day 5 to day 25 of the menstrual cycle.
Endometriosis:
10 mg two or three times daily from day 5 to day 24 of the cycle or continuously.
Dysfunctional bleeding (to arrest bleeding):
10 mg twice daily for five to seven days. Duphaston should be given with oestrogen.
Dysfunctional bleeding (to prevent bleeding):
10 mg twice daily from day 11 to day 25 of the cycle.
Duphaston should be given with oestrogen.
Amenorrhoea:
An oestergen once daily from day 1 to day 25 of the cycle. Together with 10 mg dydrogesterone twice daily from day 11 to day 25 of the cycle.
Irregular cycles:
10mg twice daily from day 11 to day 25 of the cycle.
Threatened abortion:
40 mg at once, then 10 mg every eight hours until symptoms remit.
Habitual abortion:
10 mg twice daily until the twintieth week of pregnancy.
Infertility due to luteal insufficiency:
10 mg daily from day 14 to day 25 of the cycle. The treatment should be continued for at least 6 consecutive cycles. It is advisable to continue this treatment during the first months of any pregnancy using the doses stated with respect to habitual abortion.
There is no relevant use of dydrogesterone before menarche. The safety and efficacy of dydrogesterone in adolescents aged 12 to 18 years has not been established. Currently available data are described in section 4.8 and 5.1, but no recommendation on a posology can be made.
-
ហាមប្រើ
• Known hypersensitivity to the active substance or to any of the excipients.
• Known or suspected progestogen dependent neoplasms (e.g. meningioma)
• Undiagnosed vaginal bleeding
• Contraindications for the use of estrogens when used in combination with dydrogesterone.
• Existence of serious liver disorders, or serious liver disorders in the medical history as long as the liver function values have not normalised.
-
ផលរំខាន
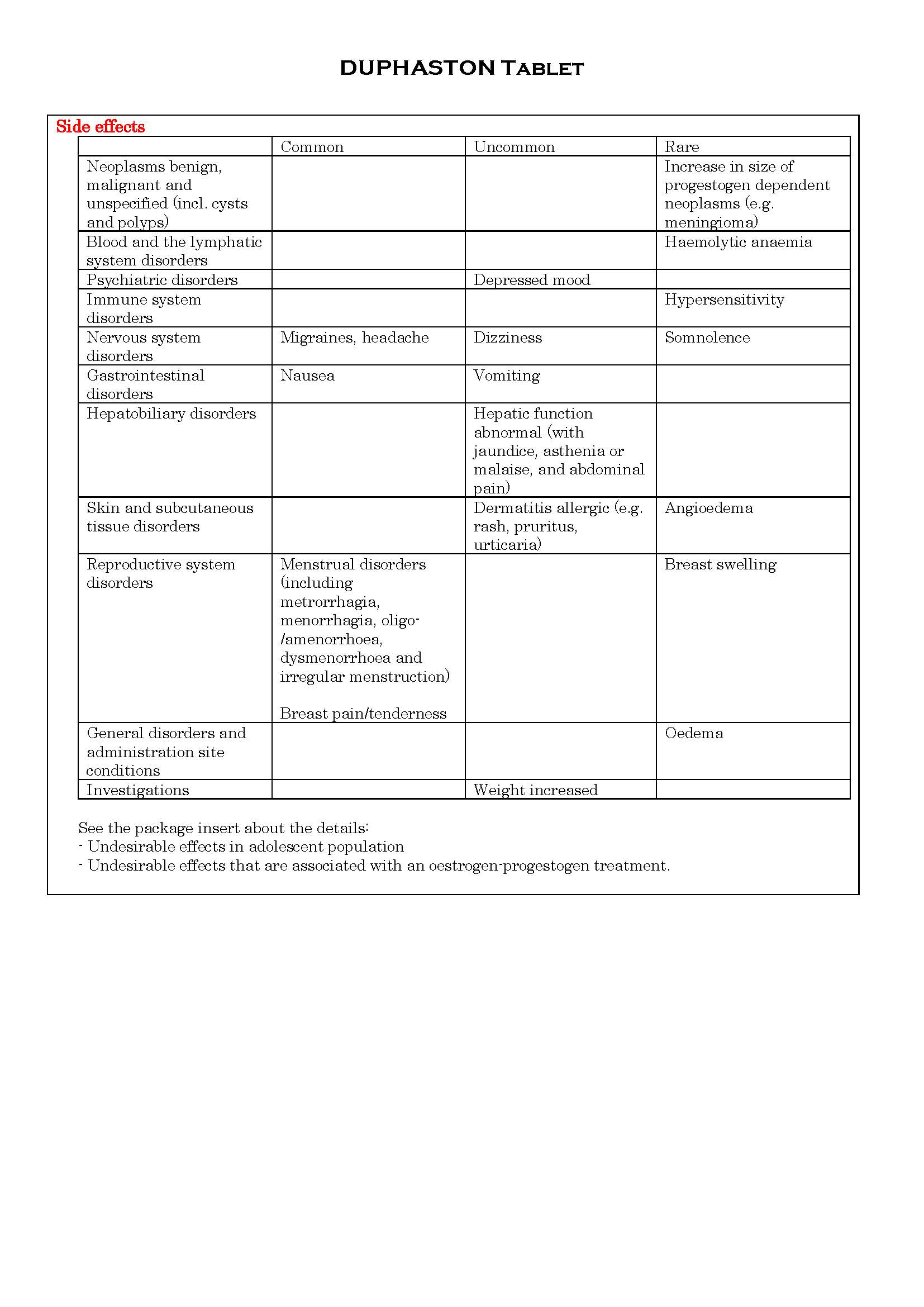
-
អន្តរប្រតិកម្ម
In vitro data show that the major metabolic pathway generating the main pharmacologically active metabolite 20α dihydrodydrogesterone (DHD) is catalyzed by aldo-keto reductase 1C (AKR 1C) in human cytosol. Next to the cytosolic metabolism there are metabolic transformations by cytochrome P450 isoenzymes (CYPs), nearly exclusively via CYP3A4, resulting in several minor metabolites. The main active metabolite DHD is substrate for metabolic transformation by CYP3A4.
Therefore, the metabolism of dydrogesterone and DHD may be increased by concomitant use of substances known to induce these CYP enzymes such as anticonvulsants (e.g. Phenobarbital, phenytoin, carbamazepine), anti-infectives (e.g. rifampicin, rifabutin, nevirapine, efavirenz) and herbal preparations containing e.g. St John's Wort (Hypericum perforatum), sage, or gingko biloba.
Ritonavir and nelfinavir, although known as strong cytochrome enzyme inhibitors, by contrast exhibit enzyme-inducing properties when used concomitantly with steroid hormones.
Clinically, increased metabolism of dydrogesterone may lead to a decreased effect and changes in the bleeding pattern.
In vitro studies have shown that dydrogesterone and DHD do not inhibit or induce GYP drug-metabolizing enzymes at clinically relevant concentrations.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
It is estimated that more than 10 million pregnancies have been exposed to dydrogesterone. So far there were no indications of a harmful effect of dydrogesterone use during pregnancy.
Some progestogens have been reported in literature to be associated with an increased risk of hypospdias. However due to confounding factors during pregnancy, no definitive conclusion can be drawn regarding the contribution of progestogens to hypospadias. Clinical studies, where a limited number of women were treated with dydrogesterone early in pregnancy, have not shown any increase in risk. No other epidemiological data are hitherto available.
Effects in non-clinical embryo-fetal and post-natal development studies were in line with the pharmacological profile. Untoward effects occurred only at exposures which exceeded the maximum human exposure considerably, indicating little relevance to clinical use.
Dydrogesterone can be used during pregnancy if clearly indicated.
Breastfeeding:
No data exist on excretion of dydrogesterone in mother's milk. Experience with other progestogens indicates that progestogens and the metabolites pass to mother's milk in small quantities. Whether there is a risk to the child is not known. Therefore, dydrogesterone should not be used during the lactation period.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Before initiating dydrogesterone treatment for dysfunctional uterine bleeding, an organic cause must be ruled out.
Breakthrough bleeding and spotting may occur during the first months of treatment. If breakthrough bleeding or spotting appears after some time on therapy, or continues after treatment has been discontinued, The reason should be investigated. If necessary by endometrial biopsy to exclude endometrial malignancy.
If any of the following disorders occurs for the first time or worsens during use, discontinuation of the treatment must be considered:
- Exceptionally severe headache, migraine or symptoms which might suggest cerebral ischaemia
- A notable rise in blood pressure
- Occurrence of venous thromboembolism
In cases of habitual and threatened abortion, the viability of the foetus should be determined and checked during whether the embryo is still alive.
Conditions which need monitoring
It is known that the following rare conditions can be influenced by sex hormones, and during pregnancy or during the use of sex hormones may occur or worsen: cholestatic jaundice, herpes gestationis, severe pruritus, otosclerosis and porphyria.
Patients with a history of depression should be monitored carefully, if severe depression recurs, the treatment with dydrogesterone must be stopped.
Other conditions
Patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
See the package insert about the details below:
- The following warnings and precautions apply when using dydrogesterone in combination with oestrogens for hormone replacement therapy (HRT);
For the treatment of postmenopausal symptoms, HRT should be initiated only if these symptoms adversely affect quality of life. In all cases, a careful appraisal of the risks and benefits should be undertaken at least annually and the treatment should be continued only if the benefit outweighs the risks.
- Medical examination/ follow-up
- Endometrial hyperplasia and carcinoma
- Breast cancer
- Ovarian cancer
- Venous thromboembolism
- Coronary artery disease
- Ischaemic stroke
-
សកម្មភាពឱសថ
Dydrogesterone is an orally-active progestogen which produces a complete secretory endometrium in an oestrogen-primed uterus thereby providing protection against the increased risk for endometrium hyperplasia and/ or carcinogenesis induced by oestrogens. It is indicated in all cases of endogenous progesterone deficiency. Dydrogesterone has no oestrogenic, no androgenic, no thermogenic, no anabolic and no corticoid activity.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
