ASOLAN Tablet
ក្រុមហ៊ុនផលិតឱសថ:
DUOPHARMA (M) SDN BHD, Malaysia
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
1. ASOLAN Tablet 0.25mg:
Alprazolam 0.25mg
2. ASOLAN Tablet 0.5mg:
Alprazolam 0.5mg
3. ASOLAN Tablet 1mg:
Alprazolam 1mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
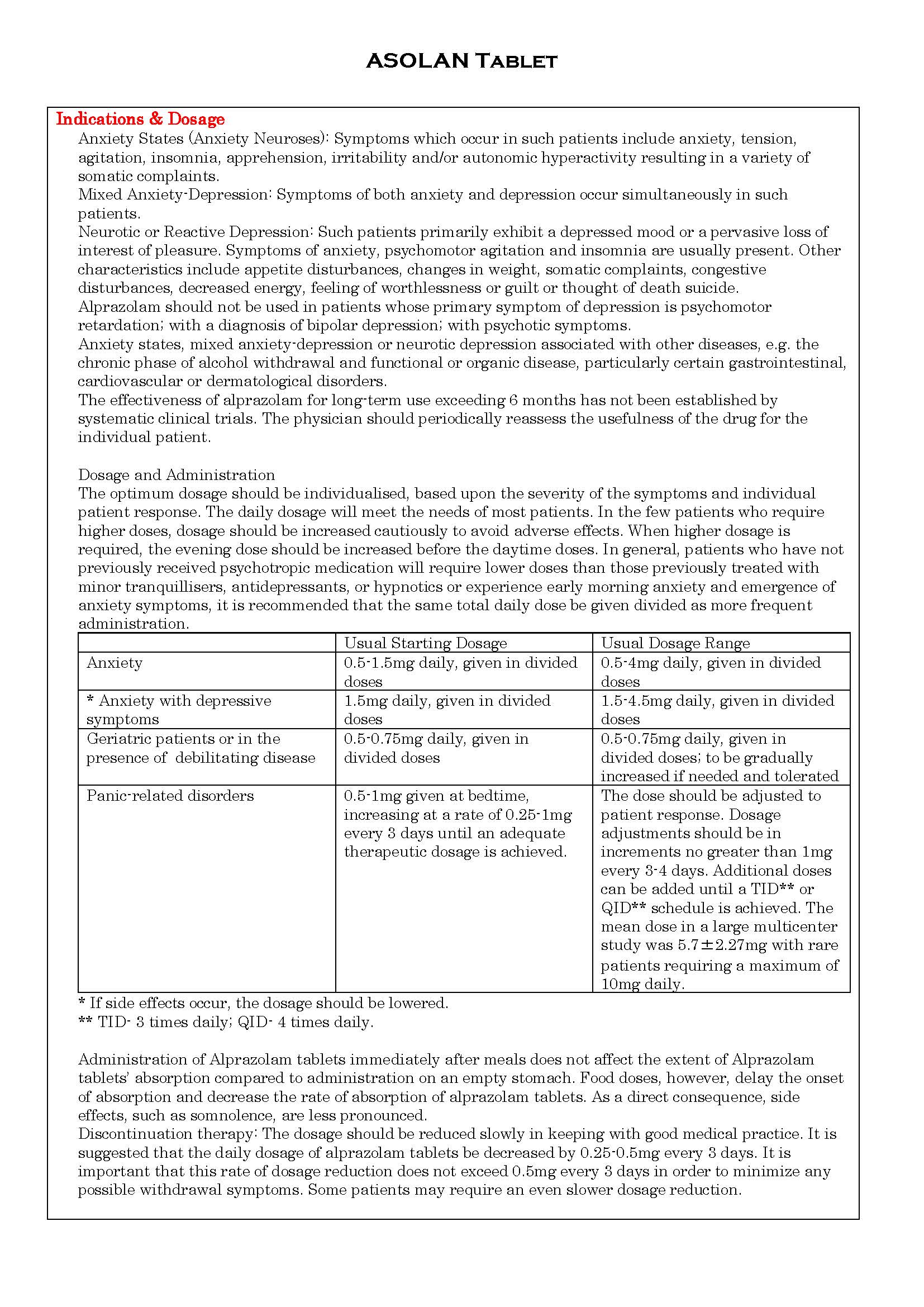
-
ហាមប្រើ
Hypersensitivity to benzodiazepines; myasthenia gravis; chronic obstructive airways disease with incipient respiratory failure.
-
ផលរំខាន
Side effects are generally observed at the beginning of therapy and usually disappear upon continued medication or decreased dosage. In patients treated for anxiety associated with depression, the most common adverse reactions to alprazolam tablets were drowsiness and lightheadedness/dizziness. Less common adverse reactions were blurred vision, headache, depression, insomnia, nervousness/anxiety, tremor, change in weight, memory impairment/amnesia, coordination disorders, various gastrointestinal symptoms, and autonomic manifestations. As with other benzodiazepines, reactions such as stimulation, agitation, concentration difficulties, confusion, hallucinations, or other adverse behavioural effects, have been reported with alprazolam. Increased intra-ocular pressure has been rarely reported.
In addition, the following adverse events have been reported in association with the use of anxiolytic benzodiazepines, including alprazolam; dystonia, irritability, anorexia, fatigue, slurred speech, jaundice, musculoskeletal weakness, changes in libido, menstrual irregularities, incontinence, urinary retention, and abnormal liver function.
The most common adverse reactions in patients with panic related disorders were sedation/drowsiness; fatigue, ataxia/impaired coordination and slurred speech. Less common adverse reactions were altered mood, GI symptoms, dermatitis, memory problems, sexual dysfunction, intellectual impairment and confusion.
Rarely, jaundice or abnormal liver function tests occur during alprazolam therapy, with recovery of function after cessation of use.
Episodes of hypomania, mania and other adverse behavioural effects may occur in rare instances with the use of alprazolam, and may necessitate the discontinuation of therapy. Such discontinuation should follow the recommended daily dosage reduction regimen.
-
អន្តរប្រតិកម្ម
The benzodiazepines including alprazolam tablets, produce additive CNS depressant effects when co-administered with drugs such as barbiturates, alcohol, sedatives, tricyclic antidepressants, non-selective MAO inhibitors and other antipsychotics, skeletal muscle relaxants, antihistamines, narcotic analgesics and anaesthetics.
Alprazolam undergoes oxidative metabolism, and consequently may interact with disulfiram or cimetidine resulting in increased plasma levels. Increased levels of alprazolam have also been observed with co-administration of erythromycin. Patients should be observed closely for evidence of enhanced benzodiazepine repose during concomitant treatment with disulfiram, cimetidine, erythromycin or other macrolide antibiotics; some patients may require a reduction in dosage of Alprazolam.
The anticholinergic effects of other drugs, including atropine and similar drugs, antihistamines and antidepressants may be potentiated when taken in conjunction with benzodiazepines.
Interactions have been reported between some benzodiazepines and anticonvulsants, with changes in the serum concentration of the benzodiazepine or the anticonvulsant. It is recommended that patients be observed for altered responses when benzodiazepines and anticonvulsants are prescribed together, and that serum level monitoring of the anticonvulsant be performed more frequently. Minor EEG changes usually low voltage fast activity, of no known clinical significance, has been reported with benzodiazepine administration.
The steady state plasma concentrations of imipramine and desipramine have been reported to be increased an average of 31% and 20% respectively, by the concomitant administration of alprazolam tablets in doses up to 4mg/day. The clinical significance of these changes is unknown.
Alprazolam causes a small decrease (7%) in lithium clearance. Caution should be exercised with the close monitoring of lithium concentrations to avoid toxicity.
Oral contraceptives may increase the elimination half-life of alprazolam: a 20% increase in the alprazolam steady-state plasma concentration may be expected in women taking alprazolam tablets and oral contraceptives concurrently.
Interaction with anti-HIV drugs: Ritonavir: A significant decrease in mean alprazolam Cmax values (16%) but not in mean AUC values (12%). Similarly, a significant effect was observed on the sedation effect curve but not on the extent of sedation. Mild psychomotor impairment was confounded by a learning effect. These pharmacokinetic and pharmacodynamic results are inconsistent with considering the pharmacologic effect of alprazolam. These results were not considered clinically significant.
Indinavir: Competitive inhibition of the metabolism of these drugs and create the potential for serious and or life-threatening adverse events such as prolonged sedation or respiratory depression.
Amprenavir: Serum concentrations may increased which could increase the activity of alprazolam. Although specific studies have not been performed, co-administration with amprenavir should be avoided due to the potential for prolonged sedation.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
Benzodiazepines cross the placenta and may cause hypotonia, respiratory depression and hypothermia in the newborn infant. Continuous treatment during pregnancy and administration of high doses in connection with delivery should be avoided. Withdrawal symptoms in newborn infants have been reported with this class of drug.
Lactation
Human studies on the excretion of alprazolam in breast milk have not been performed. Studies in mice, however, indicated that alprazolam and its metabolites are excreted in breast milk.
Benzodiazepines generally show increased toxicity in neonates, and the excretion of benzodiazepines in breast milk may cause drowsiness and/or feeding difficulties in the infant. Therefore, unless there are compelling circumstances to the contrary, alprazolam is not recommended for use while breast-feeding.
-
ការប្រុងប្រយ័ត្នជាពិសេស
This preparation may be habit forming on prolonged use.
As with all patients taking CNS-depressant medication, patients receiving Alprazolam should be warned not to operate dangerous machinery or motor vehicles until it is known that they do not become drowsy to dizzy from alprazolam therapy. Abilities may be impaired on the day following use. Patients should be advised that their tolerance for alcohol and other CNS depressants will be administered and that these medications should either be eliminated or given in reduced dosage in the presence of alprazolam.
Anaphylaxis (severe allergic reaction) and angioedema (severe facial swelling), which can occur as early as the first time the product is taken.
Complex Sleep-Related behaviors which may include sleep driving, making phone calls, preparing and eating food (while asleep).
Following the prolonged use of Alprazolam at therapeutic doses, withdrawal from the medication should be gradual. An individualized withdrawal timetable needs to be planned for each patient in whom dependence is known or suspected (periods from 4 weeks to 4 months have been suggested). As with other benzodiazepines, when treatment is suddenly withdrawn, a temporary increase in sleep disturbance can occur after use of Alprazolam.
In general, benzodiazepines should be prescribed for short periods only (e.g. 2-4 weeks). With the exception of the use of Alprazolam for the treatment of Panic Disorder, continuous long-term use of alprazolam is not recommended. There is evidence that tolerance develops to the sedative effects of benzodiazepines. After as little as 1 week therapy with recommended doses, withdrawal symptoms can appear following cessation of treatment, e.g. rebound anxiety following the cessation of an anxiolytic benzodiazepine.
(See the package insert about the details below.)
Depression, Psychosis and Schizophrenia
Paradoxical Reactions
Geriatric of Debilitated patients
Hypotension
Epilepsy
Impaired renal/liver function
Impaired respiratory function
Acute Narrow-angle glaucoma
Amnesia
Abuse
Withdrawal and dependence
Use in paediatrics
-
សកម្មភាពឱសថ
In general, benzodiazepines act as depressants of the central nervous system (CNS), producing all levels of CNS depression from mild sedation to hypnosis to coma depending on dose. The precise sites and mechanisms of action have not been completely established. Although various mechanisms of action have been reported, it is believed that benzodiazepines enhance or facilitate the inhibitory neurotransmitter action of gamma-amino butyric acid (GABA), which is one of the major inhibitory neurotransmitters in the brain and mediates both pre- and post-synaptic inhibition in all regions of the CNS, following interaction between the benzodiazepine and a specific neuronal membrane receptor.
Pharmacological properties of alprazolam in animals appear similar to those of other benzodiazepines, that is, it produces significant anxiolytic, muscle relaxant, sleep promoting and anticonvulsant effects in appropriate animal models.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
