ARICEPT EVESS Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Bunshu Pharmaceuticals Ltd. Misato Factory, Japan
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
1. ARICEPT EVESS 5mg:
2. ARICEPT EVESS 10mg:
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
For the symptomatic treatment of:
- mild, moderate and severe Alzheimer’s disease
- vascular dementia (dementia associated with cerebrovascular disease).
Posology and method of administration
Adults/Elderly:
Treatment is initiated at 5mg/day (once-a-day dosing).
ARICEPT EVESS should be taken orally, in the evening, just prior to retiring.
The tablet should be placed on the tongue and allowed to disintegrate before swallowing with or without water, according to patient preference. The 5mg/day dose should be maintained for at least one month in order to allow the earliest clinical responses to treatment to be assessed and to allow the earliest clinical responses to treatment to be assessed and to allow steady-state concentrations of donepezil hydrochloride to be achieved. Following a 4-6 weeks of clinical assessment in patients who tolerated treatment at 5mg/day, the dose can be increased to 10mg/day (once-a-day dosing). The maximum recommended daily dose is 10mg. Doses greater than 10mg/day have not been studied in clinical trials. Upon discontinuation of treatment, a gradual abatement of the beneficial effects of ARICEPT is seen. There is no evidence of a rebound effect after abrupt discontinuation of therapy.
Renal and hepatic impairment: See the package insert about the details.
Children
ARICEPT is not recommended for use in children.
-
ហាមប្រើ
In patients with a known hypersensitivity to donepezil hydrochloride, piperidine derivatives, or to any excipients used in the formulation.
In pregnancy.
-
ផលរំខាន
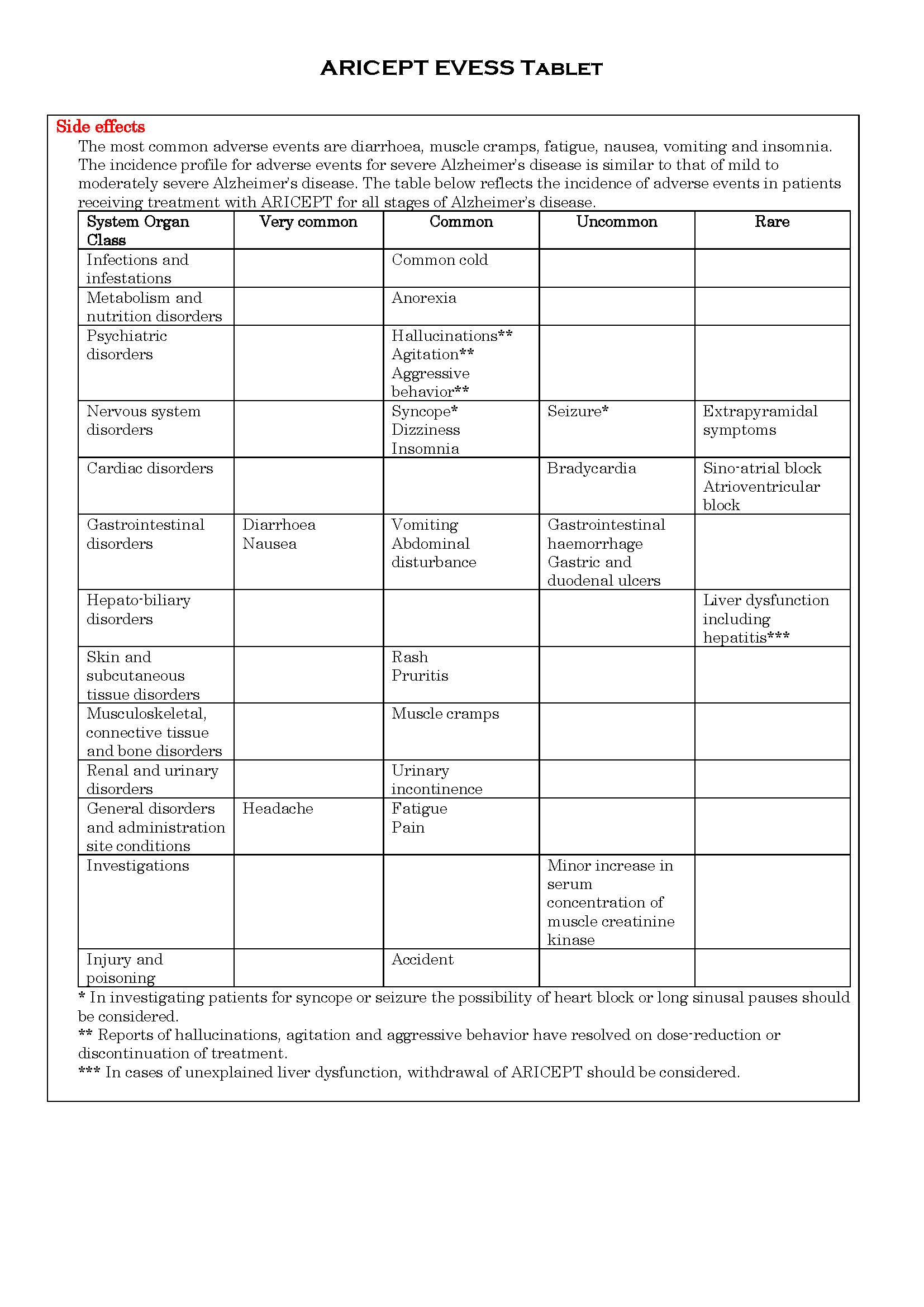
-
អន្តរប្រតិកម្ម
Donepezil hydrochloride and/or any of its metabolites do not inhibit the metabolism of theophylline, warfarin, cimetidine or digoxin in humans. The metabolism of donepezil hydrochloride is not affected by concurrent administration of digoxin or cimetidine.
In vitro studies have shown that the CYP3A4, 2D6 are involved in the metabolism of donepezil. Drug interaction studies performed in vitro show that ketoconazole and quinidine, inhibitors of CYP3A4 and 2D6 respectively, inhibit donepezil metabolism. Therefore, these and other CYP34 inhibitors, such as itraconazole and erythromycin, and CYP2D6 inhibitors, such as fluoxetine could inhibit the metabolism of donepezil.
In a study in healthy volunteers, ketoconazole increased mean donepezil concentrations by about 30%. Enzyme inducers, such as rifampicin, phenytoin carbamazepine and alcohol may reduce the levels of donepezil. Since the magnitude of an inhibition or inducing effect is unknown, such drug combinations should be used with care.
Donepezil hydrochloride has the potential to interfere with medications having anticholinergic activity. There is also the potential for synergistic activity with concomitant treatment involving medications such as succinylcholine, other neuro-muscular blocking agents or cholinergic agonists or beta blocking agents which have effects on cardiac conduction.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
See the package insert about the details below:
Pregnancy
Should not be used during pregnancy.
Lactation
Women on donepezil should not breast feed.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Treatment should be initiated by a physician experienced in the treatment of dementia. Diagnosis should be made according to accepted guidelines (e.g. DSMⅣ, ICD 10). Therapy with donepezil should only be started if a caregiver is available who will regularly monitor drug intake for the patient. Maintenance treatment can be continued for as long as a therapeutic benefit for the patient exists. Therefore, the clinical benefit of donepezil should be reassessed on a regular basis. Discontinuation should be considered when evidence of a therapeutic effect is no longer present. Individual response to donepezil cannot be predicted. The use of ARICEPT in patients with other types of dementia or other types of memory impairment (e.g. Amnestic Mild Cognitive Impairment), is under investigation.
See the package insert about the details below:
Anaesthesia
Cardiovascular
Gastrointestinal conditions
Neurological conditions
Pulmonary conditions
Severe hepatic impairment
Mortality in vascular dementia clinical trials
-
សកម្មភាពឱសថ
The Pharmacotherapeutic group: drugs for dementia:
Donepezil hydrochloride is a specific and reversible inhibitor of acetyl cholinesterase, the predominant cholinesterase in the brain.
Donepezil hydrochloride is in vitro over 1000 times more potent an inhibitor of this enzyme than of butyryl cholinesterase, and enzyme which is present mainly outside the central nervous system.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
