APUZIN Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Y.S.P.INDUSTRIES(M) SDN.BHD. Malaysia.
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
INTERMEDICA
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Captopril 25mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
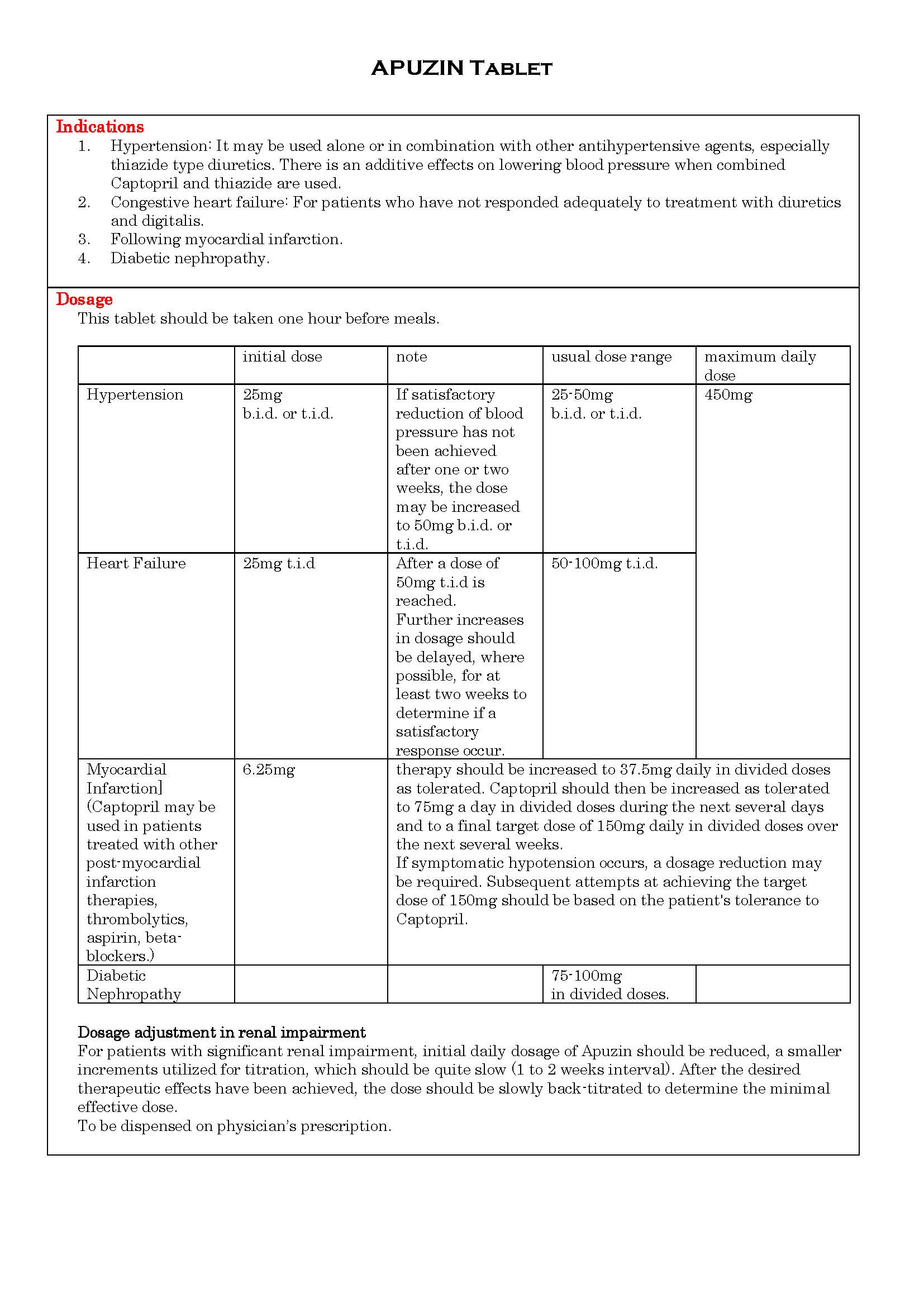
-
ហាមប្រើ
1. in patients who are hypersensitive to this product or any other angiotensin converting enzyme inhibitor.
2. in Pregnancy. It can cause fetal and neonatal morbidity and death when administered to pregnant women.
FDA Pregnancy Category C: First Trimester.
FDA Pregnancy Category D: Second and Third Trimester.
-
ផលរំខាន
Renal: Proteinuria, transient elevation of BUN and creatinine, and hyperkalemia.
Hematologic: Neutropenia
Dermatologic: Rash often with pruritus, and sometimes with fever and eosinophilia. It is usually maculopapular, and rarely urticarial during the first 4 weeks of therapy. The rash is usually mild and disappears within a few days of dosage reduction. Pemphigoid-like lesion and photosensitivity may also occur but may disappear upon discontinuing of therapy.
Cardiovascular: Hypotensive may occur in congestive heart failure, renin-dependent hypertension or serious volume depleted patients.
Gastrointestinal: Patients develop a diminution or loss of taste perception. Taste impairment is reversible or usually self-limited (2-3 months). Weight loss may be associated with the loss of taste. Some patients may develop aphthous ulcers, gastric irritation or abdominal pain.
Others: Paresthesias, serum sickness, cough, bronchospasm and lymphadenhypertrophy.
-
អន្តរប្រតិកម្ម
1. Concurrent administration of potassium sparing diuretic with ACE inhibitors may result in hyperkalemia.
2. ACE inhibitor may interact with other antihypertensive to produce additive hypotensive effects.
3. Concurrent use of NSAIDs, especially indomethacin may antagonize the antihypertensive effects of ACE inhibitors.
4. Captopril may reduce excretion of lithium, causing increased plasma-lithium concentration.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Contraindicated in Pregnancy. It can cause fetal and neonatal morbidity and death when administered to pregnant women.
FDA Pregnancy Category C: First Trimester.
FDA Pregnancy Category D: Second and Third Trimester.
-
ការប្រុងប្រយ័ត្នជាពិសេស
1. Proteinuria may occur by the eighth month of therapy especially in patients with a history of renal disease. In most cases, proteinuria subsides or clear within 6 months whether or not Captopril is continued. In patients who develop proteinuria exceeding 1g/day or proteinuria that is increasing, the benefits and risks of continuing Captopril should be evaluated.
2. In patients with collagen vascular disease (e.g. systemic lupus erythematous, scleroderma) and impaired renal function, neutropenia occurred in 3.7% of patients in clinicals trial. If Captopril is used in patients with impaired renal function, white blood cell and differential counts should be evaluated prior to starting treatment and at approximately 2-weeks intervals for about 3 months, then periodically.
3. All patients treated with Captopril should be told to report any signs of infections (e.g. sore throat, fever). If infection is suspected, while cell counts should be performed without delay. Discontinuation of Captopril and other drug has generally led to prompt return of the white cell count to normal.
4. Because of the potential fall in the blood pressure in patients with heart failure (with blood pressure either normal or low), therapy should be started under close medical supervision, at least one hour after the initial dose. If hypotensive occurs, the patients should be placed in supine position and, if necessary, receive an Ⅳinfusion of normal saline. This transient hypotensive response is not contraindicated to further doses which can be given without difficulty once the blood pressure has increased after volume expansion.
5. Some patients with renal diseases, particularly those with severe renal artery stenosis, have developed increased in BUN and serum creatinine after reduction of blood pressure with Captopril.
6. Captopril may caused false-positive reactions to urinary acetone and for dipstick tests for urinary ketones.
7. In patients undergoing major surgery or during anesthesia with agents that produce hypotension, Captopril blocks angiotensin Ⅱformation secondary to compensatory renin release. This may lead to hypotension which can be corrected by volume expansion.
8. Captopril is excreted in breast milk, with the concentration approximately 1% of maternal blood concentration. There is potential serious adverse reaction in the nursing infants, therefore risk-benefit of the use of captopril in nursing should be considered.
9. In pediatrics use, there is risk of oliguria and neurologic abnormalities. Safety and effectiveness in children have not been established.
-
សកម្មភាពឱសថ
1. The beneficial effects or Captopril in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin-aldosterone system. Reduction of angiotensin Ⅱleads to aldosterone secretion and, as a result, small increase in serum potassium may occur along with sodium and fluid loss. Captopril prevents the convertion of angiotensin Ⅰto angiotensin Ⅱby inhibition of ACE.
2. After oral administration of therapeutic doses of Captopril, rapid absorption occurs with peak blood levels at about one hour. The presence of food in the gastrointestinal tract reduces absorption by about 30 to 40%, Captopril therefore should be given one hour before meals.
3. Approximately 25 to 30% of the circulated drug is bound to plasma protein. The apparent elimination half-life of Captopril metabolite in blood is probably less than 3 hours. In patients with renal impairment, retention of unchanged Captopril occurs.
4. In a 24-hour period, over 95% of the absorbed dose is eliminated in the urine; 40-50% is unchanged drug; most of the remainder is the disulfide dimer of Captopril and Captopril-cysteine disulfide.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
