ANLODIN Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Y.S.P INDUSTRIES (M) SDN. BHD., Malaysia
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
INTERMEDICA


- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
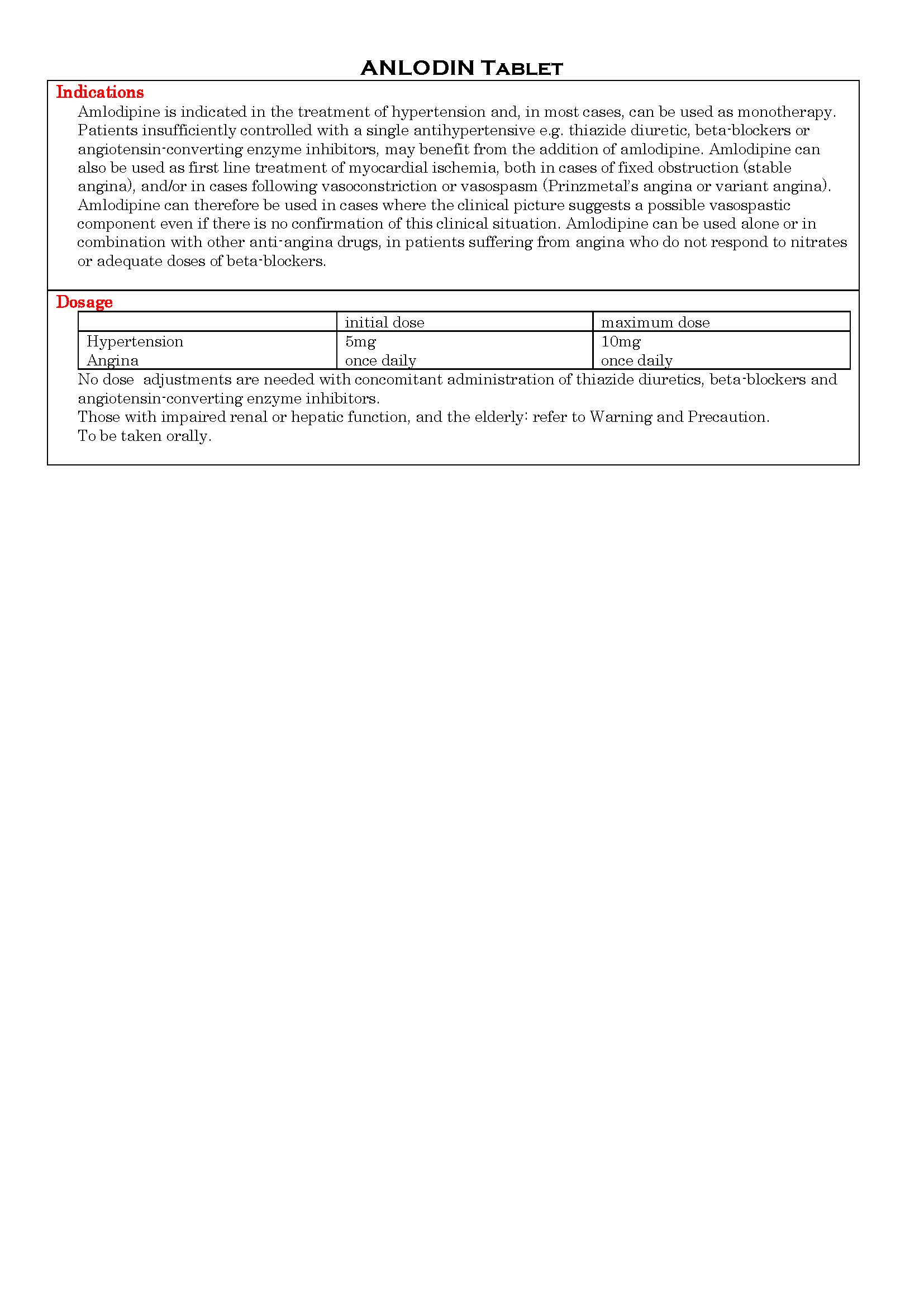
-
ហាមប្រើ
Known sensitivity to dihydropyridines.
-
ផលរំខាន
Commonly: headache, edema, fatigue, nausea, flushing, dizziness.
Less commonly: pruritus, rash, dyspnea, asthenia, muscle cramps, dyspepsia, gingival hyperplasia.
Rarely: erythema multiforme.
The following side effects are rarely reported and cannot be distinguished from the natural history of the underlying diseases, e.g. myocardial infarction, arrhythmia (including ventricular tachycardia and atrial fibrillation), chest pain.
No pattern of clinically significant laboratory test abnormalities related to amlodipine has been observed.
-
អន្តរប្រតិកម្ម
Amlodipine has been safely administered together with thiazide diuretics, beta blockers, angiotensin-converting enzyme inhibitors, long-acting nitrates, sublingual nitroglycerin, NSAIDs, antibiotics and oral hypoglycemic drugs.
In healthy male volunteers, effect of warfarin on prothrombin response time is not significantly altered by co-administration of amlodipine. Co-administration of amlodipine also did not alter serum digoxin level or digoxin clearance in normal volunteers. Co-administration of cimetidine did not alter the pharmacokinetics of amlodipine.
In vitro studies indicate that amlodipine has not effect on protein-binding of the drugs tested (digoxin, phenytoin, warfarin or indomethacin).
Incompatibilities : Not relevant.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
use in pregnacy is only recommended if tere is no safer alternative and when the disease itself carries greater risk for the mother and child.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Use in patients with impaired renal function: Amlodipine is extensively metabolized in inactive form and 10% is excreted unchanged in the urine. The degree of renal impairment is not linked to changes in the amlodipine plasma concentrations. Amlodipine can be used in this condition at normal doses. Amlodipine is not dialyzable.
Use in patients with impaired hepatic function: As with all calcium antagonists, the plasma half-life of amlodipine is prolonged in patients with impaired hepatic function. For these patients specific dosage recommendations have not been established. The drug should therefore be used with caution in these patients.
Effects on the ability to drive and operate machinery: Clinical experience with amlodipine indicates that it is unlikely to impair a patient’s ability to drive or operate machinery.
Use in the children: No data available on use of amlodipine in children.
Use in the elderly: The time needed for amlodipine to reach the peak plasma concentration in the elderly and in younger subjects is similar. However, the clearance of amlodipine tends to decrease in elderly, causing increases in the AUC and of the elimination half-life of the drug. At similar doses, amlodipine is equally well tolerated in the elderly and young patient, normal dosage regimens are recommended.
-
សកម្មភាពឱសថ
Amlodipine is a calcium ion influx inhibitor (slow channel blocker or calcium ion antagonist) and inhibits the transmembrane influx of calcium ions into cardiac and vascular smooth muscle.
The antihypertensive effect of amlodipine is due to a direct relaxant effect on vascular smooth muscle.
The precise mechanism by which amlodipine relieves angina has not been fully determined but amlodipine reduces total ischemic burden by the following actions:
1) Reduces total peripheral resistance (afterload) by dilating peripheral arterioles. Thus myocardial energy consumption and oxygen requirements are reduces since the heart rate remains stable.
2) Amlodipine may also dilate the main coronary arteries and coronary arterioles, both in normal and ischemic regions. This will increase myocardial oxygen delivery in patients with coronary artery spasm (Prinzmetal’s or variant angina) and blunt smoking induced coronary vasoconstriction.
Once daily dosing of amlodipine in hypertensive patient provides clinically significant blood pressure reductions in both the supine and standing positions throughout the 24 hours interval. Amlodipine does not case acute hypotension due to its slow onset of action.
Once daily dosing of amlodipine in patients with angina increases total exercise time, time to angina onset, and time to 1mm ST segment depression, and decreases both angina attach frequency and nitroglycerin tablet consumption.
Amlodipine did not adversely affect the metabolism and plasma lipid profile, thus is suitable to be used in patients with asthma, diabetes and gout. Amlodipine also did not after the pharmacokinetics of cyclosporine significantly.
Studies indicated that amlodipine did not lead to clinical deterioration in NYHA Class Ⅱ-Ⅳ heart failure patients measured by exercise tolerance, left ventricular ejection fraction and clinical symptomatology.
Amlodipine is well absorbed after oral administration with peak blood levels between 6-12 hours post dose. Bioavailability is about 64-80%. Plasma protein binding is around 97.5% Volume of distribution is around 21L/kg. Absorption is not affected by food.
The terminal elimination half-life is around 35-50 hours and is consistent with once daily dosing. Steady state plasma levels are reached after 7-8 days of consecutive dosing. Amlodipine is extensively metabolized to inactive metabolites with 10% of parent compound and 60% of metabolites excreted in urine.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
