AMLOCOR-5 Tablet
ក្រុមហ៊ុនផលិតឱសថ:
TORRENT PHARMACEUTICALS LTD., India
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Amlodipine 5mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
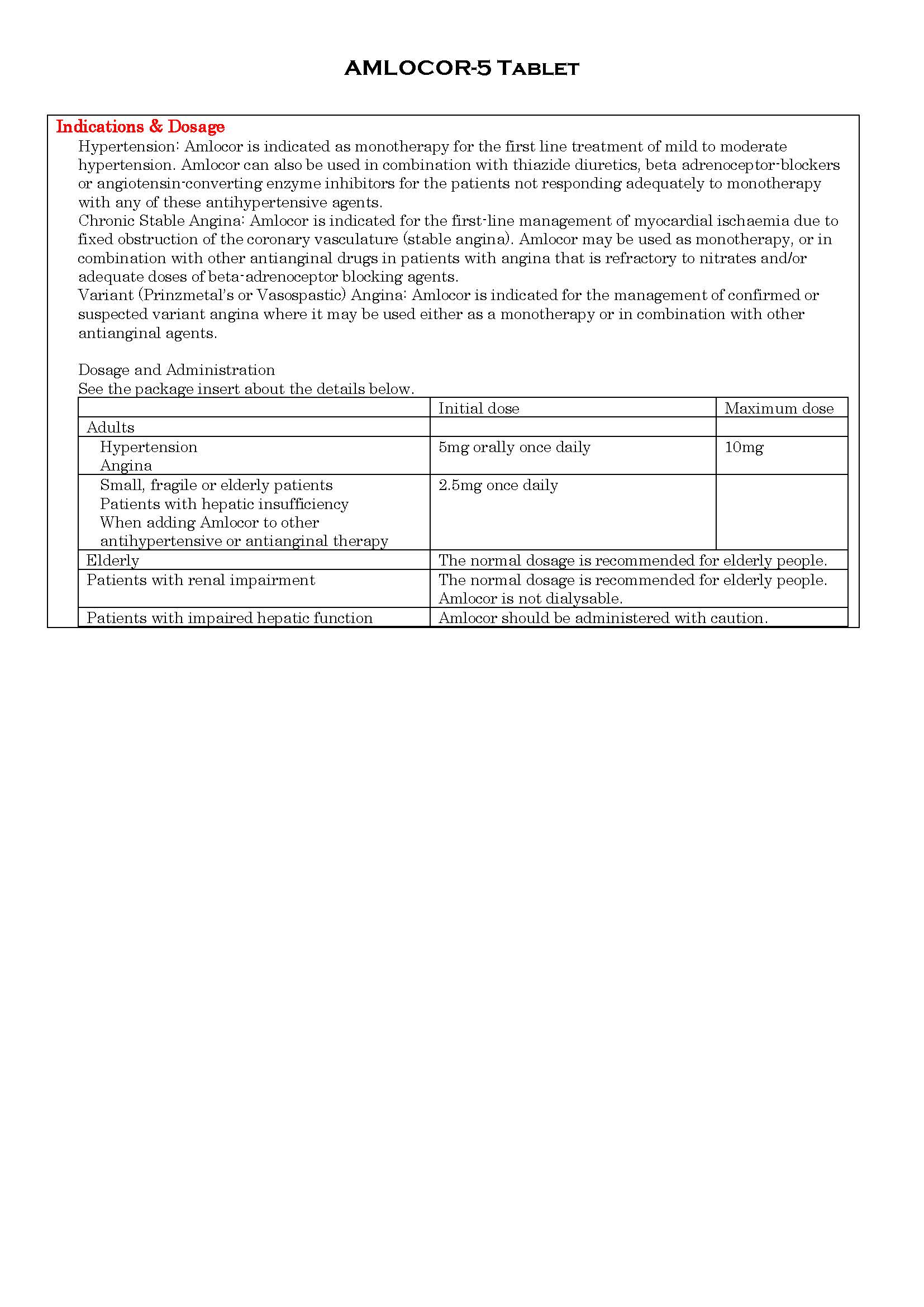
-
ហាមប្រើ
In patients with a known hypersensitivity to dihydropyridines (e.g. nifedipine, nicardipine, isradipine).
-
ផលរំខាន
The most commonly observed side effects are headache, oedema, fatigue, flushing and dizziness. Less common side effects include nausea, abdominal pain, somnolence and palpitations. Rare side effects include muscle cramps, frequency of micturition or nocturia, coughing, breathlessness, epistaxis, impotence, nervousness and conjunctivitis. No clinically significant pattern of laboratory test abnormalities related to Amlocor has been observed.
Amlocor has not been associated with any adverse metabolic effects or changes in plasma lipids. Amlocor has been used safely in patients with well compensated congestive heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, abnormal lipid profiles and diabetes mellitus.
-
អន្តរប្រតិកម្ម
Amlocor has been safely administered with thiazide diuretics, beta-adrenoceptor blocking drugs, angiotensin-converting enzyme inhibitors, long-acting nitrates, sublingual glyceryl trinitrate, NSAIDs, antibiotics, and oral hypoglycaemic agents.
Co-administration of Amlocor with digoxin did not change serum digoxin levels or digoxin renal clearance in normal volunteers.
Co-administration of cimetidine did not alter the pharmacokinetics of Amlocor.
In healthy volunteers, co-administration of Amlocor did not significantly alter the effect of warfarin on prothrombin time. The introduction of Amlocor is not likely to result in the need for modification of an established warfarin regimen.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
There is no clinical experience with Amlocor in pregnancy or lactation. Amlocor should not be administered during pregnancy or lactation or to women of child-bearing potential unless effective contraception is ensured. Since there is no clinical experience, use of Amlocor is not currently recommended for children and adolescents of less than 18 years of age.
-
ការប្រុងប្រយ័ត្នជាពិសេស
The half-life of amlodipine is prolonged in patients with impaired liver function; Amlocor should be administered with caution in such patients. Amlocor should be administered with caution in patient receiving either peripheral vasodilators (especially in patients with severe aortic stenosis) or in patients with severe heart failure.
-
សកម្មភាពឱសថ
Amlocor acts as a calcium ion antagonist in peripheral vascular and coronary smooth muscle cells; thus producing marked vasodilatation in peripheral and coronary vascular beds. The reduction in peripheral blood pressure produced by Amlocor is a result of peripheral arterial vasodilatation resulting in a reduction of peripheral vascular resistance. In patients with mild to moderate essential hypertension, Amlocor has a sustained and gradual onset of antihypertensive effect. Amlocor, in once daily dosage regimens of 2.5-10mg has been shown to produce reductions in mean systolic and diastolic pressures of about 10-18% in hypertensive patients. Moreover, in such patients Amlocor also increased renal blood flow as well as glomerular filtration rate, and reduced renovascular resistance. Urine volume and urinary sodium excretion were unchanged suggesting that Amlocor has no long-term effect on sodium homeostasis. Plasma renin activity as well as aldosterone and catecholamine levels were not significantly affected.
Amlocor reduces myocardial oxygen demand, increases myocardial oxygen supply and improves exercise capacity in patients with symptomatic myocardial ischaemia. In patients with stable angina pectoris, Amlocor, through reduction in peripheral vascular resistance, reduces afterload as well as the rate-pressure product, thereby reducing myocardial oxygen demand. Amlocor also inhibits coronary spasm and restores coronary blood flow in patients of vasospastic (Prinzmetal’s) angina.
Amlocor has no significant effects on the sinoatrial or atrioventricular node. In clinical studies where Amlocor was administered concomitantly with beta-blockers to patients with either hypertension or angina, no adverse effects on electrocardiographic parameters were observed.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
