AMLOBOSTON 5 Tablet
ក្រុមហ៊ុនផលិតឱសថ:
BOSTON PHARMACEUTICAL Inc., USA
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Amlodipine 5mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់

-
ហាមប្រើ
- Hypersensitivity to dihydropyridine derivatives, amlodipine or to any of the excipients.
- Severe hypotension.
- Shock (including cardiogenic shock).
- Obstruction of the outflow tract of the left ventricle (e.g. high grade aortic stenosis).
- Haemodynamically unstable heart failure after acute myocardial infarction.
-
ផលរំខាន
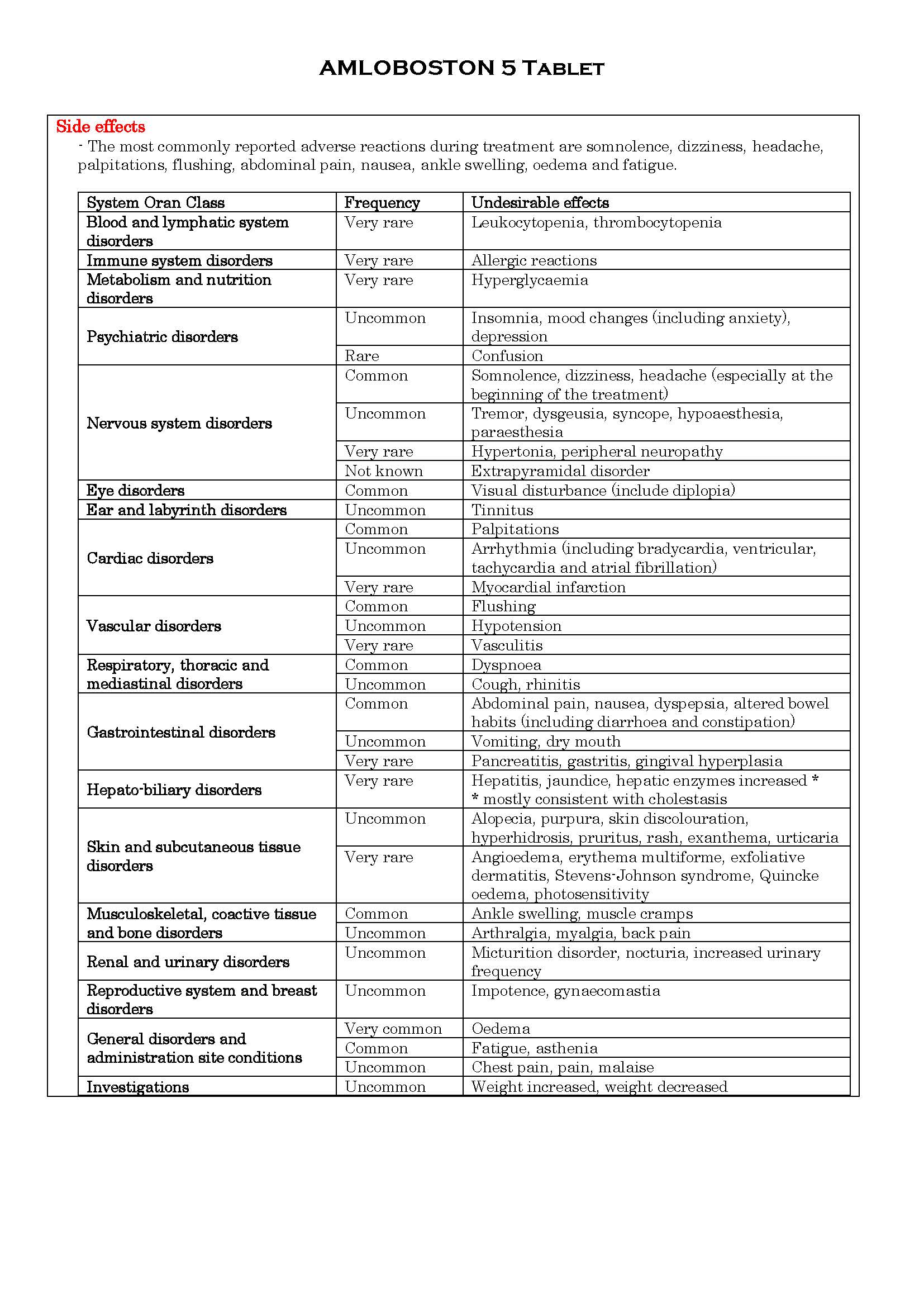
-
អន្តរប្រតិកម្ម
See the package insert about the details below:
Effects of other medicinal products on amlodipine
- CYP3A4 inhibitors (protease inhibitors, azole antifungals, macrolides like erythromycin or clarithromycin, verapamil or diltiazem)
- CYP3A4 inducers (rifampicin, hypericum perforatum) (Grapefruit or grapefruit juice)(Dantrolene (infusion))
Effects of amlodipine on other medicinal products
- Medical products with antihypertensive properties.
- Atorvastatin, digoxin, warfarin or cyclosporin.
- Simvastatin
- Tacrolimus
- Ciclosporin
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
- The safety in human pregnancy has not been established.
- Use in pregnancy is only recommended when there is no safer alternative and when the disease itself carries greater risk for the mother and foetus.
Lactation
It is not known whether amlodipine is excreted in breast milk. A decision on whether to continue/discontinue breast-feeding or to continue/discontinue therapy with amlodipine should be made taking into account the benefit of breast-feeding to the child and the benefit of amlodipine therapy to the mother.
-
ការប្រុងប្រយ័ត្នជាពិសេស
The safety and efficacy of amlodipine in hypertensive crisis has not been established.
(See the package insert about the details below.)
- Patients with cardiac failure
- Hepatic impairment
- Elderly
- Renal impairment.
-
សកម្មភាពឱសថ
- Amlodipine is a calcium ion influx inhibitor of the dihydropyridine group (slow channel blocker or calcium ion antagonist) and inhibits the transmembrane influx of calcium ions into cardiac and vascular smooth muscle.
- The mechanism of the antihypertensive action of amlodipine is due to a direct relaxant effect on vascular smooth muscle.
- The precise mechanism by which amlodipine relieves angina has not been fully determined but amlodipine reduces total ischaemic burden by the following two actions:
+ Amlodipine dilates peripheral arterioles and thus, reduces the total peripheral resistance (afterload) against which the heart works. Since the heart rate remains stable, this unloading of the heart reduces myocardial energy consumption and oxygen requirements.
+ The mechanism of action of amlodipine also probably involves dilatation of the main coronary arteries and coronary arterioles, both in normal and ischaemic regions. This dilatation increases myocardial oxygen delivery in patients with coronary artery spasm (Prinzmetal’s or variant angina).
- In patients with hypertension, once daily dosing provides clinically significant reductions of blood pressure in both the supine and standing positions throughout the 24 hour interval. Due to the slow onset of action, acute hypotension is not a feature of amlodipine administration.
- In patients with angina, once daily administration of amlodipine increases total exercise time, time to angina onset, and time to 1mmST segment depression, and decreases both angina attack frequency and glyceryl trinitrate tablet consumption.
- Amlodipine has not been associated with any adverse metabolic effects or changes in plasma lipids and is suitable for use in patients with asthma, diabetes, and gout.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
