ADALAT LA Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Bayer Pharma AG, Germany
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
1. Treatment of coronary heart disease
- Chronic stable angina pectoris (angina of effort)
2. Treatment of hypertension
Dosage and Method of administration
Oral use
As far as possible the treatment must be tailored to the needs of the individual.
Depending on the clinical picture in each case, the basic dose must be introduced gradually.
Unless otherwise prescribed, the following dosage guidelines are recommended for adults:
1. For coronary heart disease:
- Chronic stable angina pectoris (angina of effort) 20-60mg once daily.
2. For hypertension: 20-60mg once daily.
In general, therapy should be initiated with 30mg once daily.
Where registered a starting dose of 20mg once daily may be considered when medically indicated. Interim doses i.e. 40mg, 50mg, etc. can be applied by combinations of i.e. 20mg+20mg or 20mg+30mg tablets.
Depending on the severity of the disease and the patient’s response the dose can be increased in stages to 12omg once daily.
Coadministration with CYP3A4 inhibitors or CYP3A4 inducers may result in the recommendation to adapt the nifedipine dose or not to use nifedipine at all (see “interactions with other medicinal products and other forms of interaction”).
Duration of Treatment
The attending doctor will determine the duration of use.
Administration
The tablets must not be chewed or broken up
As a rule the tablets are swallowed whole with a little liquid, irrespective of meal times.
Grapefruit juice is to be avoided (see “interactions with other medicinal products and other forms of interaction”).
Additional information on special populations (See the package insert about the details below.)
- Pediatric Patients
- Geriatric patients
- Patients with hepatic impairment
- Patients with renal impairment
-
ហាមប្រើ
Adalat LA must not be used in cases of known hypersensitivity to nifedipine or to any of the excipients.
Nifedipine must not be used during pregnancy and breastfeeding.
Adalat LA must not be used in cases of cardiovascular shock.
Adalat LA must not be used in patients with Kock pouch (ileostomy after proctocolectomy).
Nifedipine must not be used in combination with rifampicin because no efficient plasma levels of nifedipine may be obtained due to enzyme induction.
-
ផលរំខាន
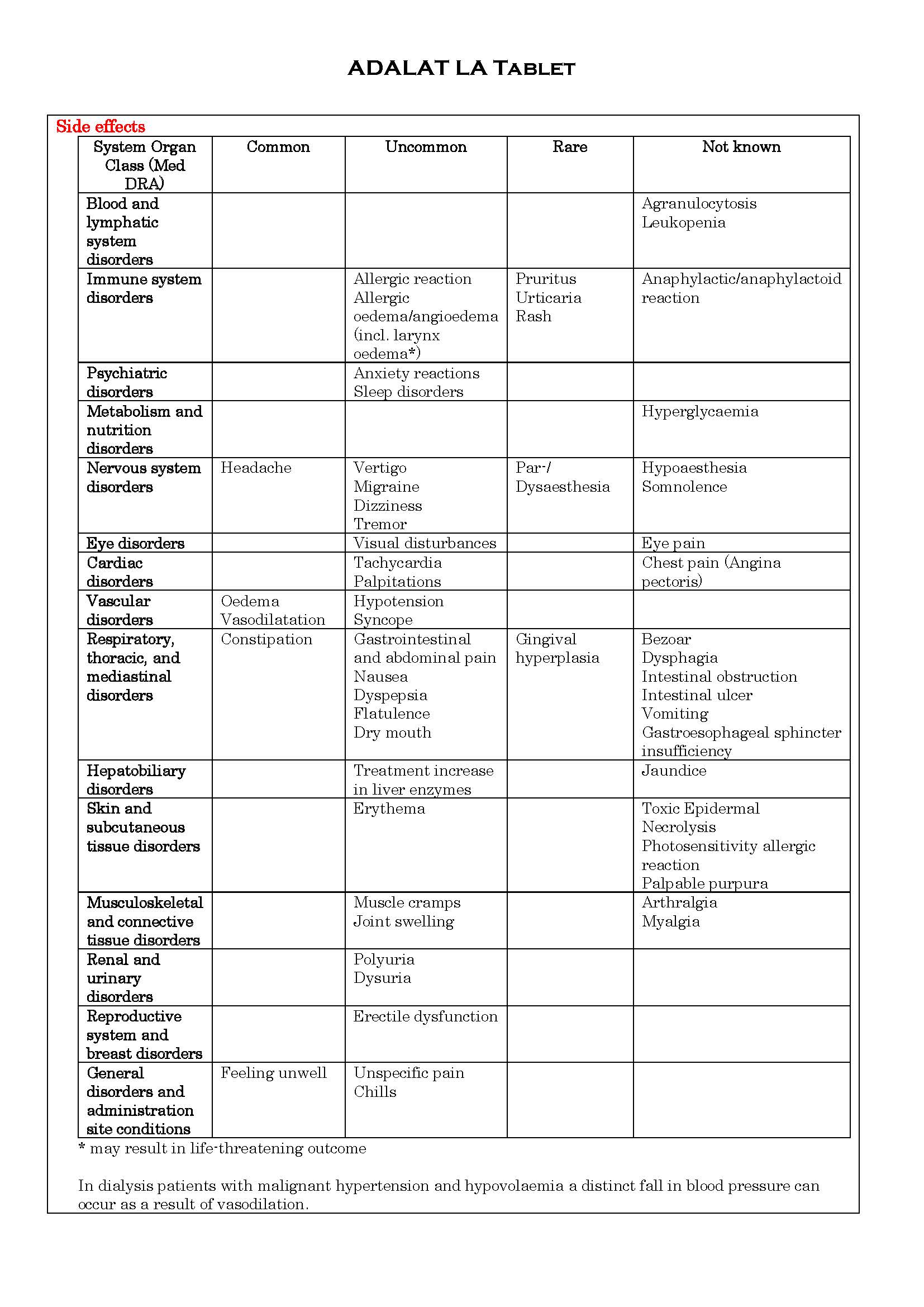
-
អន្តរប្រតិកម្ម
See the package insert about the details.
- Drugs that affect nifedipine
- Rifampicin
- Macrolide antibiotics (e.g. erythromycin)
- Anti-HIV protease inhibitors (e.g. ritonavir)
- Azole anti-mycotics (e.g. ketoconazole)
- Fluoxetine
- Nefazodone
- Quinupristin/Dalfopristin
- Valproic acid
- Cimetidine
- Cisapride
- CYP3A4 system-inducing anti-epileptic drugs, such as phenytoin, carbamazepine and phenobarbitone
- Blood pressure lowing dugs
- Digoxin
- Quinidine
- Tacrolimus
- Diltiazem
- Grapefruit juice
- Ajmaline
- Aspirin
- Benazepril
- Candesartan Cilexetil
- Debrisoquine
- Doxazosin
- Irbesartan
- Omeprazole
- Orlistat
- Pantoprazole
- Ranitidine
- Tailnolol
- Triamterene Hydrochlorothiazide
- Other forms of interaction
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Contraindicated (see the package insert about the details.)
-
ការប្រុងប្រយ័ត្នជាពិសេស
Care must be exercised in patients with very low blood pressure (severe hypotension with systolic pressure less than 90mmHg), in cases of manifest heart failure and in the case of severe aortic stenosis.
As with other vasoactive substances, angina pectoris attacks may occur in single cases at the start of the treatment with nifedipine. The occurrence of myocardial infarction has been described in single cases, although it was not possible to distinguish this from the natural course of the underlying disease.
Careful monitoring of blood pressure must be exercised, also when administered nifedipine with i.v. magnesium sulphate, owing to the possibility of an excessive fall in blood pressure which could harm both mother and fetus (see “Contraindications”).
As with other non-deformable material (see “Instructions for use/handling”) care should be used when administering Adalat LA in patients with pre-existing severe gastrointestinal narrowing because obstructive symptoms may occur. Bezoars can occur in very rare cases and may require surgical intervention.
In single cases obstructive symptoms have been described without known history of gastrointestinal disorders.
When doing barium contrast X-ray Adalat LA may cause false positive effects (e.g. filling defects interpreted as polyp).
In patients with mild, moderate or severe impaired liver function, careful monitoring and a dose reduction may be necessary. The pharmacokinetics of Nifedipine has not been investigated in patients with severe hepatic impairment (see “Dosage and method of administration” and “Pharmacokinetic properties). Therefore, Nifedipine should be used with caution in patients with severe hepatic impairment.
Nifedipine is metabolised via the CYP3A4 system. Drugs that are known to either inhibit or to induce this enzyme system may therefore alter the first pass or the clearance of nifedipine (see “Interaction with other medicinal products and other forms of interactions”).
Drugs, which are inhibitors of the CYP3A4 system and therefore may lead to increased plasma concentrations of nifedipine are, e.g.:
- macrolide antibiotics (e.g, erythromycin),
- anti-HIV protease inhibitors (e.g. ritonavir),
- azole anti-mycotics (e.g. ketoconazole),
- the antidepressants nefazodone and fluoxetine,
- quinupristin/dalfopristin,
- valproic acid,
- cimetidine.
Upon co-administration with these drugs the blood pressure should be monitored and. if necessary, a reduction of the nifedipine dose should be considered.
Dose titration up to the maximal daily dose of 120mg nifedipine may result in a maximal uptake of 2mmol sodium per day. To be taken into consideration by patients on a controlled sodium diet.
Effects on ability to drive and use machines
See the package insert.
-
សកម្មភាពឱសថ
Nifedipine is a calcium antagonist of the 1,4-dihydropyridine type. Calcium antagonists reduce the transmembranal influx of calcium ions through the slow calcium channel into the cell.
Nifedipine acts particularly on the cells of the myocardium and the smooth muscle cells of the coronary arteries and the peripheral resistance vessels.
In the heart, nifedipine dilates the coronary arteries, especially the large conductance vessels, even in the free wall segment of partially stenosed areas. Further, nifedipine reduces the vascular smooth muscle tone in the coronary arteries and prevents vasospasm. The end-result is an increased poststernotic blood flow and an increased oxygen supply. Parallel to this, nifedipine reduces the oxygen requirement by lowering peripheral resistance (afterload). With long-term use, nifedipine can also prevent the development of new atherosclerotic lesions in the coronary arteries.
Nifedipine reduces the smooth muscle tone of the arterioles, thus lowering the increased peripheral resistance and consequently the blood pressure. At the beginning of the nifedipine treatment there may be a transient reflex increase in heart rate and thus in the cardiac output. However, this increase is not enough to compensate or the vasodilation. In addition, nifedipine increases sodium and water excretion both in the short-term and long-term use. The blood-pressure-lowering effect of nifedipine is particularly pronounced in hypertensive patients.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
