ZENTEL Tablet
ក្រុមហ៊ុនផលិតឱសថ:
SAVI PHARMACEUTICAL JOINT STOCK Co., Vietnam
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
DKSH

- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Albendazole 200mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
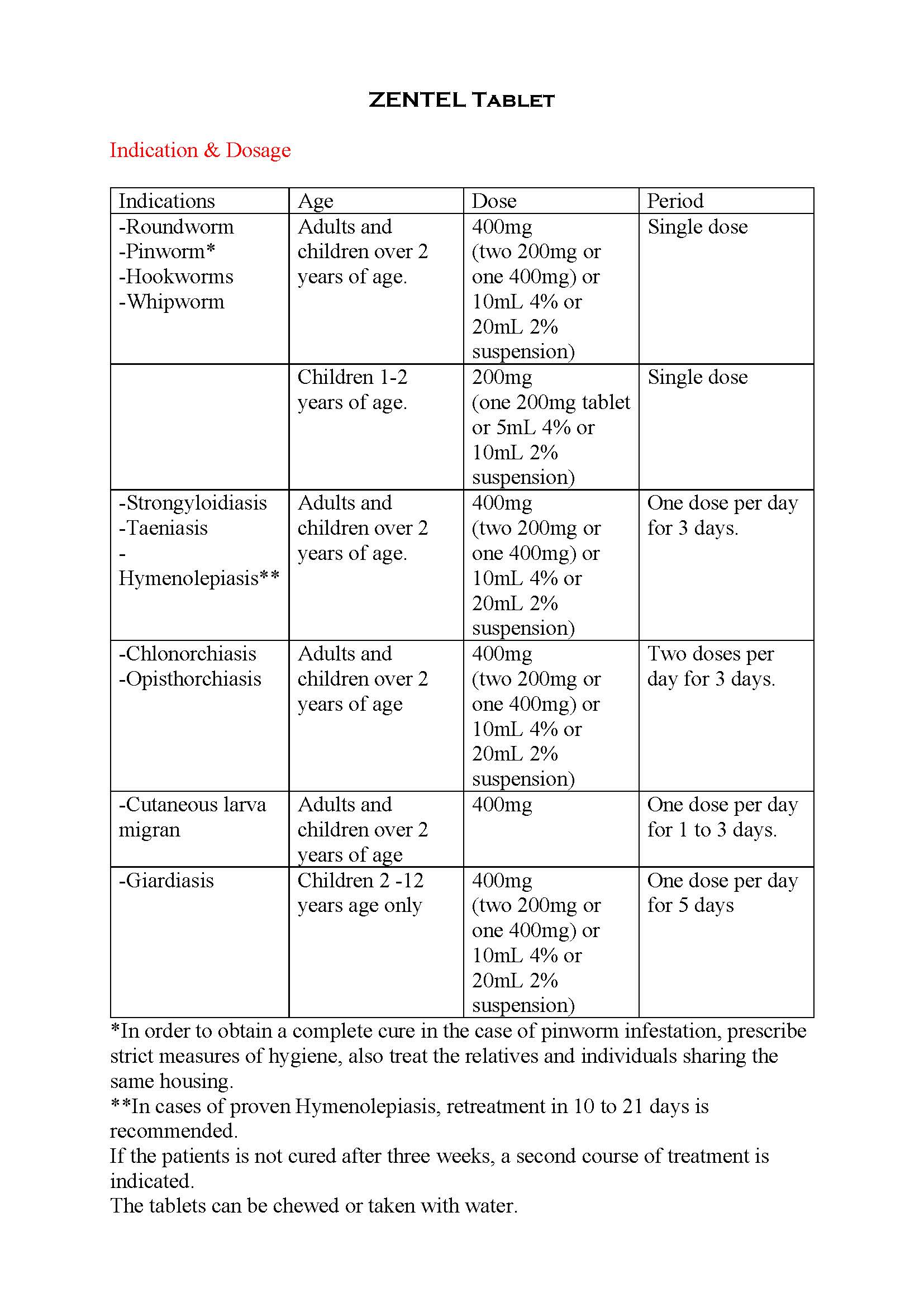
-
ហាមប្រើ
1. Pregnant patients
2. Patients with a know history of hypersensitivity to albendazole or constituents
-
ផលរំខាន
Headache, abdominal pain, nausea, vomiting, elevations of hepatic enzymes.
-
អន្តរប្រតិកម្ម
Praziquantel, ritonavir, phenytoin, carbamazepine, phenobarbital
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy: Contraindication
Lactation:Not adequate study
-
ការប្រុងប្រយ័ត្នជាពិសេស
Special populations
- Elderly
Experience in patients 65 years of age or older is limited. Reported indicate that no dosage adjustment is required, however, ZENTEL should be used with caution in elderly patients with evidence of hepatic dysfunction.
- Renal impairment
Since renal elimination of albendazole and its primary metabolite, albendazole sulfoxide, is negligible, it is unlikely that clearance of these compounds would be altered in these patients. No dosage adjustment is required, however, patients with evidence of renal impairment should be carefully monitored.
- Hepatic impairment
Since albendazole is rapidly metabolized by the liver to the primary pharmacologically active metabolite, albendazole sulfoxide, hepatic impairment would be expected to have significant effects on the pharmacokinetics of albendazole sulfoxide. Patients with abnormal liver function test results (transaminases) prior to commencing albendazole therapy should be carefully monitored.
Special warning and precautions for use
In order to avoid administering ZENTEL during early pregnancy, women of childbearing age should initiate treatment during the first week of menstruation or after a negative pregnancy test.
Treatment with ZENTEL may uncover pre-existing neurocysticercosis, particularly in areas with high taenosis infection. Patients may experience neurological symptoms e.g. seizures, increased intracranial pressure and focal signs as a result of an inflammatory reaction caused by death of the parasite within the brain. Symptoms may occur soon after treatment, appropriate steroid and anticonvulsant therapy should be started immediately.
ZENTEL suspension contains benzoic acid which is a mild irritant to the skin, eyes and mucous membrane. It may increase the risk of jaundice in newborn babies.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
