CIPRO-DENK 500 Tablet
ក្រុមហ៊ុនផលិតឱសថ:
Denk Pharma GmbH & Co.KG, Germany
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Ciprofloxacin 500mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
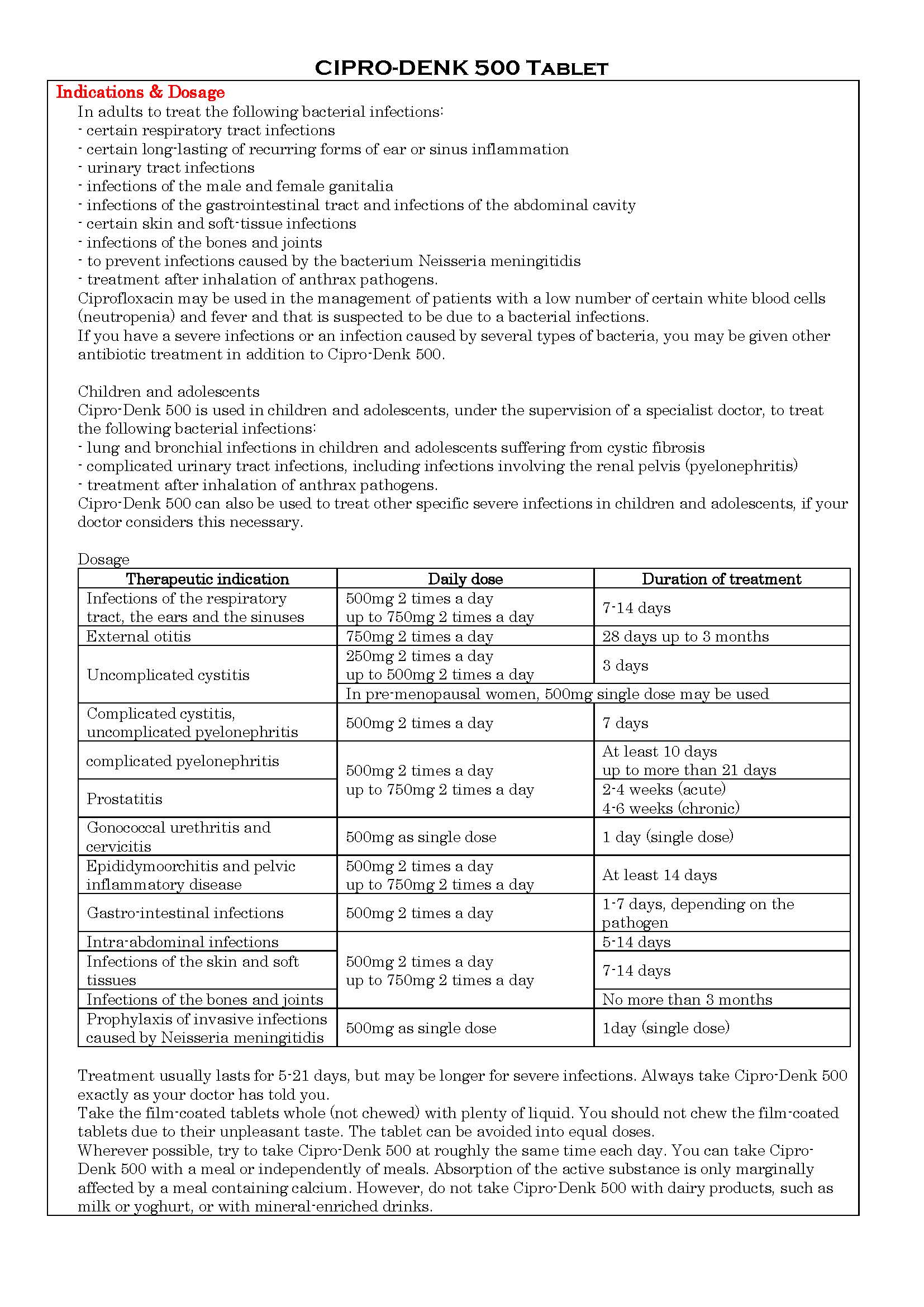
-
ហាមប្រើ
- if you are allergic to ciprofloxacin, other quinolone preparations or any other ingredients of this medicine
- if you are taking tizanidine
-
ផលរំខាន
Common
- nausea, diarrhoea
- joint pain in children
Uncommon
- fungal super infections
- high concentration of eosinophil granulocytes, certain white blood cells
- loss of appetite (anorexia)
- hyperactivity or restlessness
- headache, light-headedness, sleeping or taste disorders
- vomiting, stomach ache, digestive disorders such as upset stomach (feeling full/heart burn) or wind
- increase in certain substances in the blood (transaminases, alkaline phosphatase and/or bilirubin)
- rash, itching or nettle rash
- joint pain in adults
- muscle and bone pain(e.g. pain of the limbs, back pain, chest pain), generally feeling unwell (asthenia) or fever
- kidney dysfunction
Rare
- inflammation of the large bowel lining triggered by antibiotics (colitis), very rarely with a fatal outcome
- changes in the blood picture (leukocytopenia, leukocytosis, neutropenia, anaemia), decrease or increase in a certain blood coagulation factor (platelets and prothrombin)
- allergic reaction, swelling (oedema) or rapid swelling of the skin and mucous membranes (angioedema)
- increase in blood sugar (hyperglycaemia)
- confusion, disorientation, anxiety, nightmares, depressions (potentially leading to suicidal thoughts, suicide attempts or completed suicide) or hallucinations
- tingling, unusual sensitivity to sensory stimuli, reduced sensitivity of the skin, tremor, seizures including status epilepticus (prolonged state of confusion or excitement) or dizziness
- visual disturbances, including double vision
- ringing in the ears (tinnitus) or hearing loss or reduced hearing
- racing heart (tachycardia)
- widening of the blood vessels (vasodilation), low blood pressure or fainting
- shortness of breath, including asthmatic symptoms
- liver dysfunction, jaundice (bile accumulation) or liver inflammation
- sensitivity to light
- muscle pain, inflamed joints, increased muscle tension or cramps
- kidney failure, blood or crystals in the urine, inflammation of the urinary tract
- fluid retention or excessive sweating
- increased levels of the amylase enzyme
Very rare
- reduced number of certain red blood cells (haemolytic anaemia), a dangerous decrease in certain white blood cells (agranulocytosis), possibly life-threatening decrease in certain red and white blood cells and platelets (pancytopenia) and reduced bone marrow function, which can also be life-threatening
- severe allergic reactions (anaphylactic reaction or anaphylactic shock, with a fatal outcome-serum sickness)
- psychiatric disorders (psychotic reactions, potentially leading to suicidal thoughts, suicide attempts or a completed suicide)
- migraine, impaired coordination, unsteady waling (impaired gait), impaired sense of smell (olfactory disorder), abnormally heightened sensitivity to sensory stimuli, increased pressure inside the skull (cerebral pressure)
- problems in colour vision
- inflammation of the blood vessel walls (vasculitis)
- inflammation of the pancreas (pancreatitis)
- liver cell death (liver necrosis), very rarely progressing to life-threatening liver failure
- small, pinpoint bleeding into the skin (petechiae); various skin changes and types of rash(e.g. Stevens-Johnson syndrome or toxic epidermal necrolysis, which is sometimes fatal)
- muscle weakness, inflamed tendons, tendon rupture - especially of the large tendon at the back of the ankle (Achilles tendon)
Not known
- complaints of the nervous system, such as pain, burning, tingling, light-headedness and/or weakness of the limbs
- abnormal fast heart rhythm, life-threatening irregular heart rhythm, alteration of the heart rhythm (called “prolongation of QT interval”, seen on ECG, electrical activity of the heart)
- pustular rash
- impairment of blood clotting (in patients. treated with vitamin K antagonists)
-
អន្តរប្រតិកម្ម
Together with tizanidine, as this may cause side effects such as low blood pressure and sleepiness.
The following medicines are known to cause interactions with Cipro-Denk 500 in your body. If Cipro-Denk 500 is taken together with these medicines, the therapeutic effect of these medicines may be impaired. The probability of developing side effects may also be increased.
Please tell your doctor if you are taking any of the following medicines:
- vitamin K antagonists (e.g. warfarin) or other oral anticoagulants (blood thinners)
- probenecid
- methotrexate
- theophylline
- tizanidine
- olanzapine
- ropinirole
- phenytoin
- metoclopramide
- cyclosporin
- glibenclamide
Other medicines that can alter your heart rhythm: Medicines that can alter your heart rhythm: Medicines that belong to the group of anti-arrhythmics (e.g. quinidine, hydroquinidine, disopyramide, amiodarone, sotalol, dofetilide, ibutilide), tricyclic antidepressants, some antimicrobials (that belong to the group of macrolides), some antipsychotics.
Cipro-Denk 500 can increase the concentration of the following medicines in our blood:
- pentoxifylline
- caffeine
- duloxetine
- lidocaine
- sildenafil
Some medicines reduce the effect of Cipro-Denk 500. Please tell your doctor if you are taking or wish to take any of the following medicines:
- antacids
- omeprazole
- mineral supplements
- sucralfate
- a polymeric phosphate binder (e.g. sevelamer)
- medicines or dietary supplements containing calcium, magnesium, aluminium or iron.
With food and drink: You can take Cipro-Denk 500 with a meal or independently of meals. Absorption of the active substance is only marginally affected by a meal containing calcium. However, do not take Cipro-Denk 500 with dairy products, such as milk or yoghurt, or with mineral-enriched drinks.
Please make sure that you drink sufficient liquid during treatment with Cipro-Denk 500.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
(See the package insert about the details.)
You should preferably avoid taking Cipro-Denk 500 during pregnancy.
Do not take Cipro-Denk 500 if you are breast-feeding.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Talk to your doctor or pharmacist before taking Cipro-Denk 500.
Before you take Cipro-Denk 500
Please tell your doctor if you:
- have ever had kidney problems, as your treatment may have to be adjusted
- suffer from epilepsy or other neurological conditions such as cerebral ischemia or stroke.
- have a history of tendon problems during previous treatment with antibiotics such as Cipro-Denk 500
- suffer from myasthenia gravis (a type of muscle weakness)
- have heart problems: See the package insert about the details.
- a member of your family is known to have a deficiency in a glucose-6-phosphate dehydrogenase, as you may experience a risk of anaemia with Cipro-Denk 500.
While you take Cipro-Denk 500
Tell your doctor immediately if any of the following events occurs whilst taking Cipro-Denk 500. Your doctor will decide whether treatment with Cipro-Denk 500 needs to be stopped.
- Severe, sudden hypersensitivity reactions (anaphylactic reaction/shock, angioedema). Even after the first dose, there is a slight risk that you may experience a severe allergic reaction, which can manifest in the following symptoms: tight-chestedness, feeling dizzy, nausea or sense of imminent fainting, or feeling dizzy on standing. If this happens, stop taking Cipro-Denk 500 and consult your doctor immediately.
- Pain and swelling in the joints and tendon inflammation may occasionally occur, especially if you are elderly and are also being treated with corticosteroids. Inflammation and ruptures of tendons may occur even within the first 48 hours of treatment or up to several months after discontinuation of Cipro-Denk 500 therapy. At the first sign of pain or inflammation, stop taking Cipro-Denk 500 and rest the painful body part. Avoid any unnecessary exercise, as this can increase the risk of tendon rupture.
- Seizures or so-called status epileptics (prolonged state of confusion or excitement) can occur, especially if you suffer from epilepsy or other neurological disorders such as cerebral ischemia or stroke. If this happens, stop taking Cipro-Denk 500 and contact your doctor immediately.
- Psychiatric reactions may occur after taking the first dose of Cipro-Denk 500. If you suffer from depression or psychosis, your symptoms may get worse during treatment with Cipro-Denk 500. In rare cases, a depression or psychosis can progress to suicidal thoughts, suicide attempts or completed suicide. If this happens, stop taking Cipro-Denk 500 and contact your doctor immediately.
- Symptoms of nerve damage may occur, such as pain, burning, tingling, light-headedness and/or weakness. If this happens, stop taking Cipro-Denk 500 and contact your doctor immediately.
- Diarrhoea may occur during treatment with antibiotics, including Cipro-Denk 500, or even several weeks after you have finished treatment. If diarrhoea is severe or persistent, or if you notice that your stool contains blood or mucus, stop taking Cipro-Denk 500 immediately, as this may be life-threatening. Do not take any medicines that stop or slow down bowel movements and consult your doctor.
- Tell the doctor or laboratory staff that you are taking Cipro-Denk 500 if you have to provide a blood or urine sample.
- Please tell your doctor if you suffer from kidney problems, as your dose may need to be adjusted.
- Cipro-Denk 500 can cause liver damage. If you notice any of the following symptoms, such as loss of appetite, jaundice (yellowing of the skin), dark urine, itching, or tenderness of the stomach, stop taking Cipro-Denk 500 and consult your doctor immediately.
- Cipro-Denk 500 may lead to a reduction in the number of white blood cells and your resistance to infections may be decreased. If you experience an infections may be decreased. If you experience an infection with symptoms such as fever and a serious deterioration in your general condition, or fever together with local symptoms such as sore infections of the throat/mouth, or pain when passing water, you should consult your doctor immediately. A blood sample may show a possible reduction in white blood cells (agranulocytosis). It is important to tell your doctor about all medicines that you are taking.
- Tell your doctor if you or a member of your family is known to suffer from glucose-6-phosphate dehydrogenase deficiency, as you will be supervised by your doctor, regarding the risk of anaemia by taking Cipro-Denk 500.
- Your skin will become more sensitive to sunlight or ultraviolet light when you take Cipro-Denk 500. For this reason, avoid exposure to strong sunlight or artificial UV light such as sun beds.
- If your eyesight becomes impaired or if your eyes seem to be otherwise affected, consult an eye specialist immediately.
-
សកម្មភាពឱសថ
Cipro-Denk 500 is an antibiotic belonging to the fluoroquinolone group. The active substance is ciprofloxacin. Ciprofloxacin works by killing bacteria that cause infections. It only works in certain strains of bacteria.
As a fluoroquinolone antibiotic, ciprofloxacin has a bactericidal effect based on inhibition of topoisomeraseⅡ(DNA gyrase) and topoisomeraseⅣ. Both enzymes are needed for bacterial DNA replication, transcription, recombination and repair.
Antibacterial spectrum of efficacy
The prevalence of acquired resistances can vary for certain species with regard to geographical and temporal aspects. Knowledge of the local resistance patterns is therefore of importance, particularly in the treatment of severe infections.
If, due to the local resistance status, the use of ciprofloxacin seems questionable for at least some forms of infection, expert advice should be sought.
Commonly susceptible species are:
- Aerobic Gram-positive microorganisms like Bacillus anthracis and Staphylococcus saprophyticus,
- Aerobic Gram-negative microorganisms like Enterobacter aerogenes, Enterobacter cloaceae, Haemophilus influenzae, Moraxella catarrhalis, Neisseria meningitidis, Proteus vulgaris, Salmonella enterica (including S.typhi/parathyphi), Serratia marcescens and Shigella ssp.
- Other microorganism like Chlamydia trachomatis, Chlamydophila pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae.
Species in which acquired resistance may pose a problem during use:
Enterococcus faecalis, some subspecies of Staphylococcus aureus, and some aerobic Gram-negative microorganisms.
Inherently resistant species are:
Enterococcus faecium, Stenotrophomas maltophilia, Bacteriodes ssp., Clostridium difficile, Treponema pallidum and Ureaplasma urealyticum.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
