XYZAL Oral solution
ក្រុមហ៊ុនផលិតឱសថ:
GlaxoSmithKline, France
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Levocetrizine 500mcg/mL
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
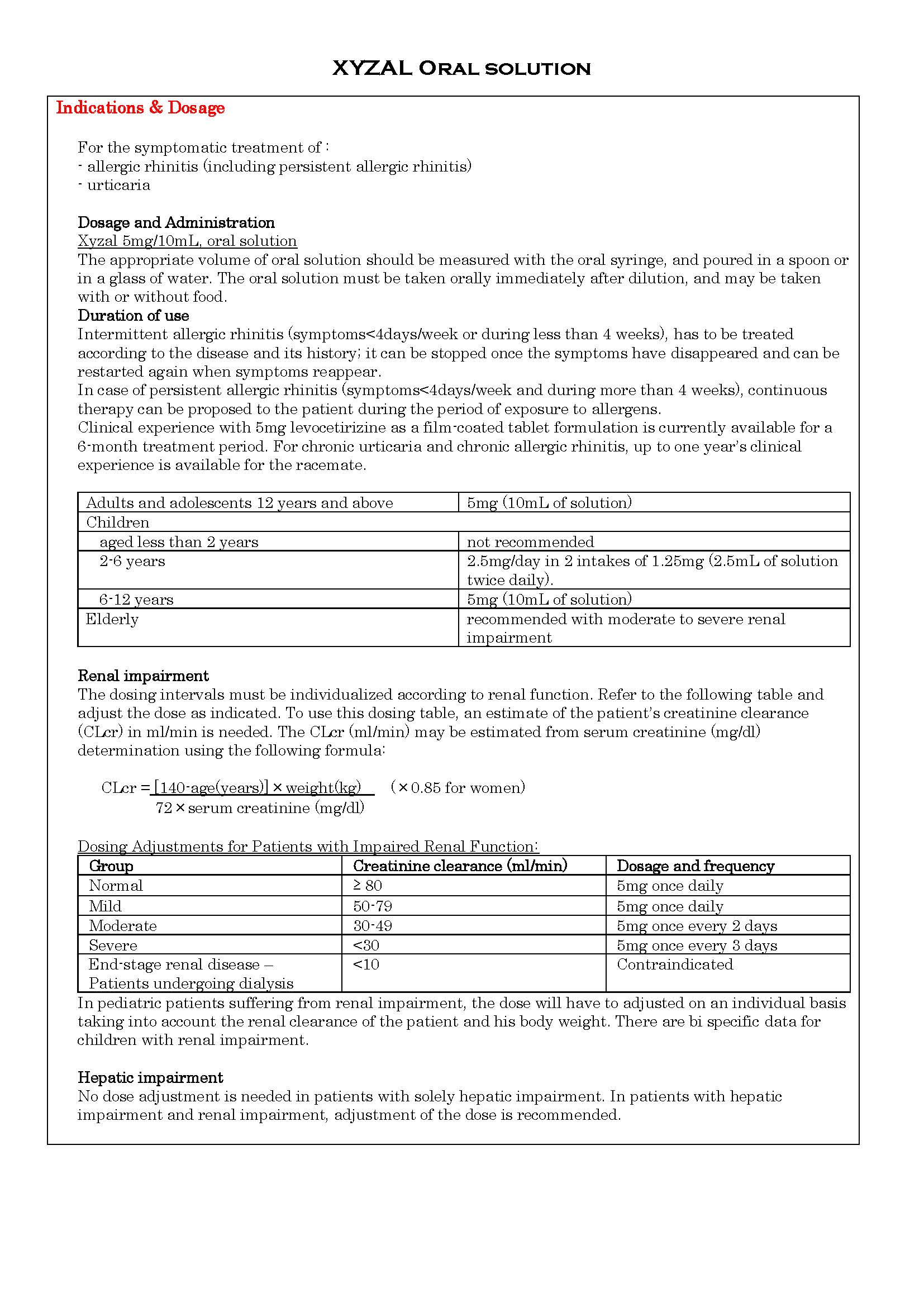
-
ហាមប្រើ
・hypersensitivity to levocetirizine, to any piperazine derivatives or any of the excitients
・severe renal impairment at less from 10 ml/min creatinine clearance
-
ផលរំខាន
Clinical Trial Data
In therapeutic studies in women and man aged 12 to 71 years, 15.1% of the patients in the levocetirizine 5mg group had at least on adverse drug reaction compared to 11.3% in the placebo group. 91.6% of these adverse drug reactions were mild to moderate.
In therapeutic included 935 subjects exposed to the drug at the recommended dose of 5mg daily.
Adverse reactions are ranked under headings of frequency using the following convention:
Very common ≥ 1/10
Common ≥ 1/100 to < 1/10
Uncommon ≥ 1/1000 to < 1/100
Rare ≥ 1/10000 to < 1/1000
Very rare < 1/10000
Not known (cannot be estimated from the available data).
Nervous system disorders
Common: headache, somnolence
Gastrointestinal disorders
Common: dry mouth
Uncommon: abdominal pain
General disorders and administration site conditions
Common: fatigue
Uncommon: asthenia
The incidence of sedating adverse drug reactions such as somnolence, fatigue and asthenia was altogether more common (8.1%) under levocetirizine 5mg than under placebo (3.1%).
Oral drops, Oral solution
Paediatric Patients
In two placebo-controlled studies aged 6-11 months and aged 1 year to less than 6 years, 159 subjects were exposed to levocetirizine at the dose of 1.25mg daily for 2 weeks and 1.25mg twice daily respectively. The following incidence of adverse drug reactions was reported under levocetirizine.
Psychiatric disorders
Common: sleep disorders
Nervous system disorders:
Common: somnolence
Gastrointestinal disorders
Common: diarrhea, constipation
Uncommon: vomiting
In children aged 6-12 years double blind placebo controlled studies were performed where 243 children were exposed to 5mg levocetirizine daily for variable periods ranging from less than 1 week to 13 weeks. The following incidence of adverse drug reactions was reported.
Nervous system disorders
Common: somnolence
Uncommon: headache
Please not that even if clinical data presented in this section are available in children aged 6 months to 12 years, we do not have sufficient data to support the administration of the product to infants and toddlers less than 2 years.
Post Marketing Data
In addition to the adverse reactions reported during clinical studies and listed above, very rare cases of the following adverse drug reactions have been reported in post-marketing experience.
Immune system disorders
Not known: hypersensitivity including anaphylaxis
Metabolism and nutrition disorders
Not known: increased weight, increased appetite
Psychiatric disorders
Not known: aggression, agitation, hallucination, depression, insomnia, suicidal ideation
Nervous system disorders
Not known: convulsions, paraesthesia, dizziness, syncope, tremor, dysgeusia
Eye disorders
Not known: Visual disturbances, blurred vision
Ear and labyrinth disorders
Not known: vertigo
Cardiac disorders
Not known: palpitations, tachycardia
Respiratory, thoracic and mediastinal disorders
Not known: dyspnea
Gastrointestinal disorders
Not known: nausea, vomiting
Hepatobiliary disorders
Not known: hepatitis, abnormal liver function test
Skin and subcutaneous tissue disorders
Not known: angioneurotic oedema, fixed drug eruption, pruritus, rash, urticaria
Musculoskeletal and connective tissue disorders
Not known: myalgia
Renal and urinary disorders
Not known: dysuria, urinary retention
General disorders and administration site conditions
Not known: oedema
-
អន្តរប្រតិកម្ម
No interaction studies have been performed with levocertirizine (including no studies with CYP34 inducers); studies with the racemate compound certirizine demonstrated that there were no clinically relevant adverse interactions(with pseudoephedrine, cimetidine, ketoconazole, erythromycin, azithromycin, glipizide and diazepam).
Theophyline
A small decrease in the clearance of cetirizine (16%) was observed in a multiple dose study with theophyline (400mg once a day); while the disposition of theophyline was not altered by concomitant cetirizine administration.
Ritonavir
In a multiple dose study of ritonavir (600mg twice a day) and cetirizine (10mg daily), the extent to cetirizine was increased by about 40% while the disposition of ritonavir was slightly altered (-11%) further to concomitant cetirizine administration.
Food
The extent of absorption of levocetirizine is not reduced with food, although the rate of absorption is decreased.
Alcohol
In sensitive patients the simultanous administration of cetirizine of levocetirizine and aldohol or other CNS depressants may have effects on the central nervous system, although it has been shown that the racemate cetirizine does not potentiate the effect of alcohol.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Fertility
There are no relevant data available.
Pregnancy
Caution should be exercised when prescribing to pregnant women.
For levocetirizine no clinical data on exposed pregnancies are avalable.
Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development, parturitaion or postnatal development.
Lactation
Caution should be exercised when prescribing to lactating women. Cetirizine is excreted inn human milk.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Alcohol
Precaution is recommended with intake of alcohol (see Section Interactions).
Risk of urinary retention
Caution should be taken in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia) as levocetirizine may increase the risk of urinary retention.
Infants and children under 2 years
Even if some clinical data are available in children aged 6 months to 12 years, these data are not sufficient to support the administration of levocetirizine to infants and toddlers aged less than 2 years.
Therefore the administration of levocetirizine to infants and toddlers aged less than 2 years is not recommended.
Oral drops
Methyl parahydroxybenzoate, propyl parahydroxybenzoate
The presence of methyl parahydroxybenzoate and propyl parahydroxybenzoate may cause allergic reactions (possibly delayed).
-
សកម្មភាពឱសថ
Antihistamine for systemic use, piperazine derivative.
levocetirizine, the (R) enantiomer of cetirizine, is a potent and selective antagonist of peripheral H1-receptors.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
