TRIATEC Tablet
ក្រុមហ៊ុនផលិតឱសថ:
PT Aventis Pharma, jakarta, Indonesia.
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
SANOFI
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
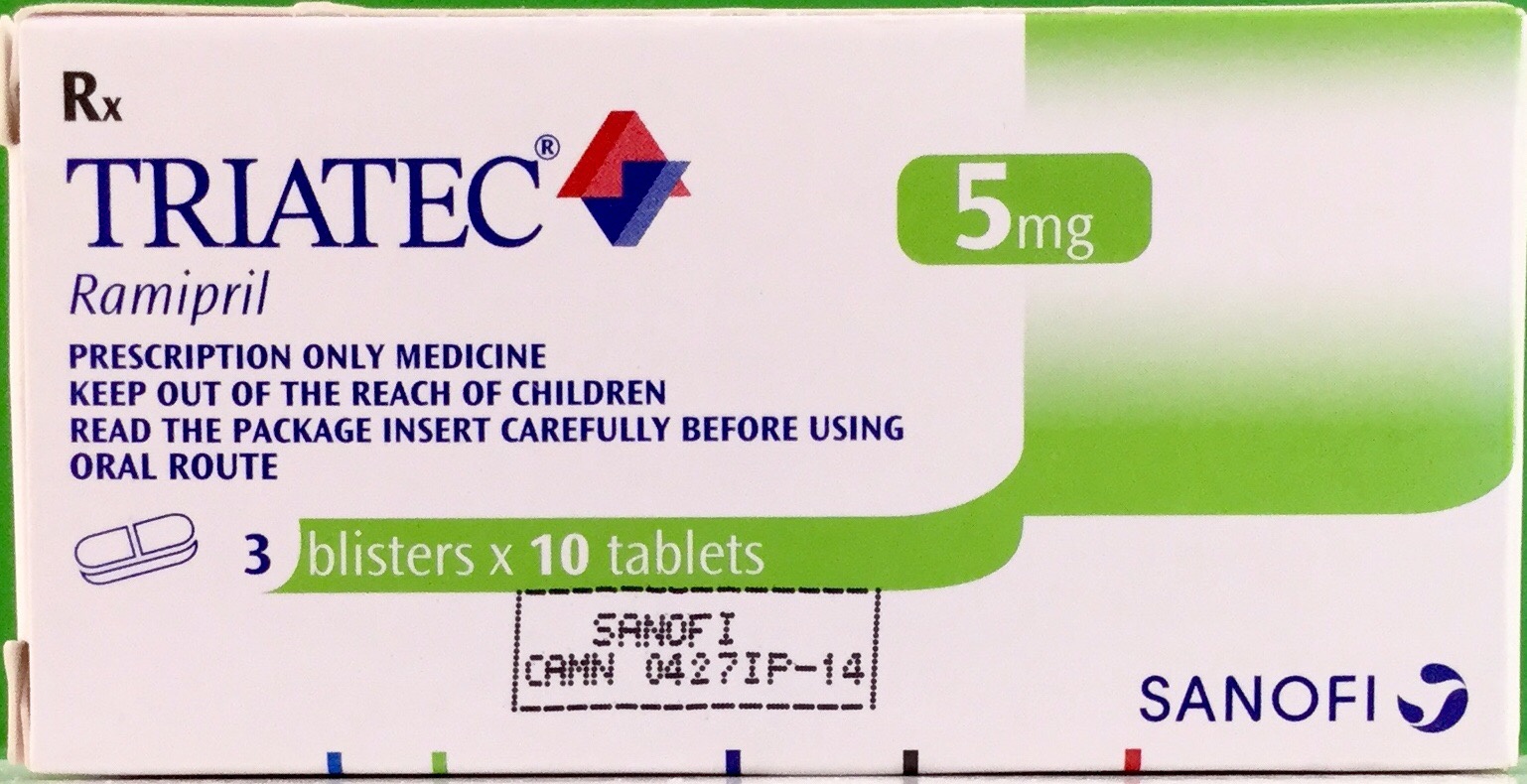
1. TRIATEC 2.5mg:
Ramipril 2.5mg
2. TRIATEC 5mg:
Ramipril 5mg
3. TRIATEC 10mg:
Ramipril 10mg
-
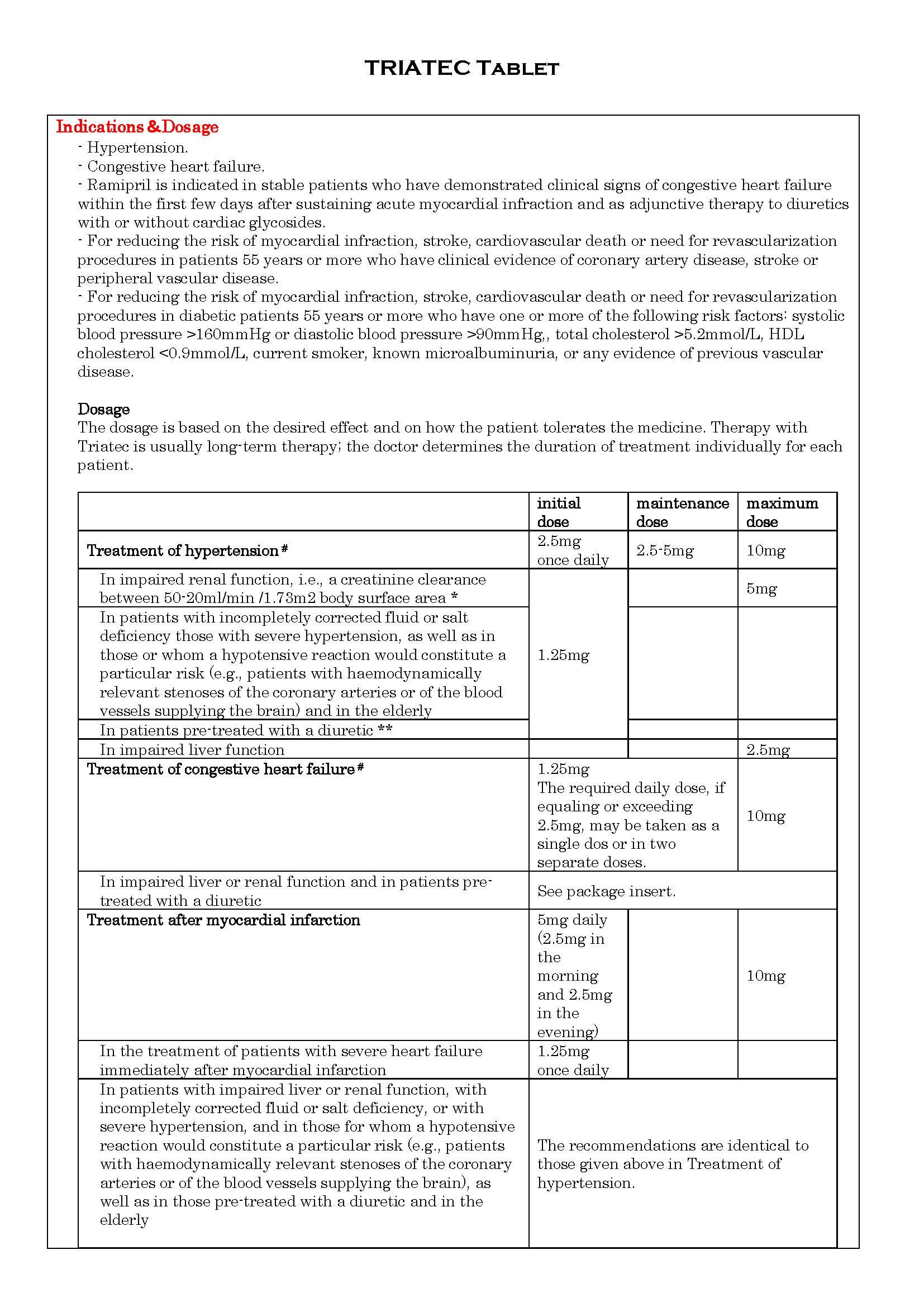
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់

-
ហាមប្រើ
- Hypersensitivity to ramipril, any other ACE inhibitor, or to any of the excipients,
- A history of angioneurotic oedema (risk of precipitating angioneurotic oedema),
- Blood-flow-reducing narrowing (haemodynamically relevant stenosis) of the renal artery, bilateral or unilateral in the single kidney (risk of fall in blood pressure and renal failure),
- In patients with low blood pressure or labile circulatory condition (risk or all in blood pressure and renal failure),
- In pregnant women, in children.
Since severe rapid-onset and allergy-like (anaphylactoid) hypersensitivity reactions may occur, treatments leading to contact of blood with negatively changed surfaces must be avoided. The latter include dialysis or haemofiltration with certain high-flux membranes (e.g. polycrylonitril membranes) ad low-density lipoprotein apheresis with dextran sulfate.
-
ផលរំខាន
Cardiovascular and nervous systems
An excessive reduction in blood pressure may occur particularly after initial or increased doses, of Triatec or of an additional diuretic, and may sometimes progress to shock.
Uncommonly, mild symptoms and reaction such as headache, disorders of balance, rapid heart rate (tachycardia), weakness, drowsiness, light-headedness or impaired reactions may occur.
Rarely, mild symptoms and reactions such as peripheral oedema, flushing, dizziness, noises in the ears (tinnitus), fatigue, nervousness, depressed mood, tremor, restlessness, visual disturbances, sleep disturbances, confusion, feeling of anxiety, transient erectile importance, palpitations, sweating, disturbed fearing, extreme sleepiness (somnolence), or feeling faint on standing or standing up (disturbed orthostatic regulation), as well as severe reactions such as angina pectoris, cardiac arrhymias and temporary loss of consciousness (syncope) may also occur.
In isolated cases, insufficient supply of blood to the heart muscle or to the brain (transient ischemic attack), heart failure, however, such corrective action must be carefully weighed ischaemic stroke, worsening of circulatory disturbances due to vascular stenosis, precipitation or intensification of attacks of circulatory disturbances characterized by, e.g. whiteness of gingers or toes (Raynauld's phenomenon) or abnormal sensations (paraesthesiae) may occur.
Kidney and electrolyte balance
Uncommonly, an increase in serum urea and serum creatinine (particularly likely in combination with diuretics), and impairment of renal function-progressing, in isolated case, to acute renal failure-may develop.
Rarely, an increase in serum potassium may occur. In isolated cases, a decrease in serum sodium may develop, as may deterioration of pre-existing pronounced urinary excretion of proteins (proteinuria) (although ACE inhibitors usually reduce proteinuria), or – in conjunction with an improvement in cardiac performance - an increase in urinary output.
Respiratory tract, anaphylactic/ anaphylactoid and skin reactions
Commonly, a dry (non-productive) tickling cough occurs. This cough is often worse at night and during periods of recumbency (i.e. white reclining), and occurs more frequently in woman and non-smokers. It may necessitate complete discontinuation of treatment with ACE inhibitors. Rarely, nasal congestion, inflammation of the nasal sinuses (sinusitis), bronchitis, spasmodic narrowing of the bronchia (bronchospasm) and difficulty in breathing (dyspnoea) may develop.
Uncommonly, the desired ACE inhibition may lead to mild angioneurotic oedema. Swelling of the facial region (e.g. lips, eyelids), or of the tongue throat, or larynx (noticeable, e.g., as difficulty in swallowing of breathing) may be signs and symptoms of such a condition. If such signs and symptoms occur, please inform your doctor immediately and stop taking the next scheduled dose of Triatec. Other ACE inhibitors must also not be used. Serious forms of angioneurotic oedema and other anaphylactic or anaphylactoid reactions not attributable to the desired effect-to ramipril or to any of the excipients, are rare. Angioneurotic oedema in conjunction with ACE inhibitors appears to occur more frequently in black, i.e. Afro-Caribbean, patients than in non-black patients.
-
អន្តរប្រតិកម្ម
- potassium-retaining diuretics (e.g., spironolactone) or with potassium salts
- antihypertensive agent (e.g., nitrates, tricyclic antidepressant, anaesthetics)
- vasopressor sympathomimetics (e.g., adrenaline, noradrenaline)
- allopurinol, immunosupressants, cytostatics, and other medicines that may alter the blood picture
- lithium
- antidiabetic agents (e.g., insulin, sulfonylurea derivatives)
- medicines for the control of pain and inflammation (NSAIDs)
- heparin
- alcohol
- anaphylactic and anaphylactoid reactions to insect venoma - and possibly, other allergens - are increased under ACE inhibition.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Triatec must not be taken during pregnancy. Therefore, pregnancy must be excluded before starting treatment. Pregnancy must be avoided where treatment with ACE inhibitors is indispensable.
If a pregnancy is planned, treatment with ACE inhibitors must be discontinued, i.e. replaced by another form of treatment. If pregnancy occurs during treatment, medication with Triatec must be substituted as soon as possible with a treatment regimen which excludes ACE inhibitors. Otherwise there is a risk of harm to the fetus. Whether exposure limited to the first 3 months of pregnancy may result in harm to the fetus is not known.
If treatment with Triatec is necessary during lactation, breast-feeding must be avoided in order to prevent the infant from ingesting small quantities of ramipril with breast milk.
-
ការប្រុងប្រយ័ត្នជាពិសេស
If angioneurotic oedema occurs during treatment, Triatec must be discontinued immediately and if the tongue, glottis or larynx are involved-emergency measures taken.
Patients with a hyper-stimulated rennin angiotensin system must be treated with particular caution. ACE inhibition puts such patients at risk of an acute pronounced fall in blood pressure and deterioration of renal function, especially when an ACE inhibitor or -in combination therapy- a medicine that promotes fluid excretion (diuretic) is given for the first time or for the first time at an increased dose. Therefore, at the start of treatment with Triatec or after the first dose of an additional diuretic, as week as after every first increased dose there of, blood pressure must be closely monitored until such time as no further acute reduction in blood pressure is expected.
Significant activation of the rennin angiotensin system is to be expected, for example, in patients:
- with severe, and particularly with malignant hypertension. The initial phase of treatment requires special medical supervision.
- with heart failure, particularly if severe or if treated with other potentially blood-pressure-lowering medicines. In severe heart failure, the initial phase of treatment requires special medical supervision.
- with blood-flow-reducing (haemodynamically relevant) left-ventricular inflow or outflowimpediment (e.g. narrowing of the aortic or mitral valve). The initial phase of treatment requires special medical supervision.
- with blood-flow-reducing narrowing (haemodynamically relevant stenosis) of the renal artery. The initial phase of treatment requires special medical supervision. Concomitant treatment with a diuretic may have to be discontinued.
- pre-treated with diuretics. Where discontinuation or reduction of the dose of the diuretic is not possible, the initial phase of treatment requires special medical supervision.
- in whom fluid or salt efficiency exist or may develop (as a result of inadequate fluid or salt intake, or as a result of, for example diarrhea, vomiting or excessive sweating in cases where salt and fluid replacement is inadequate). Generally dehydration reduced blood volume (hypovolaemia), or salt deficiency should be corrected before initiating treatment (in patient with against the risk of volume overload). When such conditions have become clinically relevant, treatment with Triatec must be started continued only if appropriate steps are taken concurrently to prevent an excessive fall in blood pressure and deterioration of renal function.
Also at particular risk from a pronounced fall in blood pressure are, e.g., patients with haemodynamically relevant stenosis of the coronary arteries or of the blood vessels supplying the brain. Such patients as well require special medical supervision in the initial phase of treatment.
In patient with impaired liver function, response to treatment with Triatec may be either increased or reduced. In addition, in patients with severe liver cirrhosis with oedema and/or ascites, the renin angiotensin system may be significantly activated: therefore, particular caution must be exercised in treating such patients.
Some elderly patients may be particularly responsive to ACE inhibitors. Renal function should be evaluated at the beginning of treatment.
Renal function should be monitored, particularly in the initial weeks of treatment. Particularly careful monitoring is required in patients with renovascular disease (including those with haemodynamically relevant unilateral renal artery stenosis, in whom even a small increase in serum creatinine may be indicative of unilateral loss of renal function), in patients with impairment of renal function, or in kidney transplant patients.
Serum potassium should be monitored regularly. More frequent monitoring of serum potassium is required in patients with impaired renal function.
The white blood cell count should be monitored so that a possible excessive reduction in white blood cells (leucopenia) can be detected. Monitoring should be more frequent in the initial phase of treatment and in patients with impaired renal function, concomitant connective tissue disease (collagen disease such as lupus erythematosus or scleroderma), or in patients treated with other medicines that may alter the blood picture. The blood picture must be checked if possible signs of reduced white blood cell or platelet counts occur.
Certain adverse effects (e.g., some symptoms of a reduction in blood pressure such as light-headedness, dizziness) may impair the ability to concentrate and react, and, therefore, constitute a risk in situations where these abilities are of particular importance (e.g., operating a vehicle or machineryl.
-
សកម្មភាពឱសថ
Ramiprilat, the active metabolite of ramipril, is a potent and long-acting angiotensin converting enzyme (ACE) inhibitor.
Ramipril is a product which, after absorption from the gastro-intestinal tract, is hydrolysed in the liver to from the active angiotensin converting enzymes (ACE) inhibitor, ramiprilat which is a potent and long acting ACE inhibitor. Administration of ramipril causes an increase in plasma renin activity and a decrease in plasma concentrations of angiotensinⅡ and aldosterone. The beneficial haemodynamic effects resulting from ACE inhibition in angiotensinⅡ causing dilatation of peripheral vessels and reduction in vascular resistance. There is evidence suggesting that tissue ACE particularly in the vasculature, rather than circulating ACE, is the primary factor determining the haemodynamic effects.
Angiotensin converting enzyme is identical with kininaseⅡ, one of the enzymes responsible for the degradation of bradykinin. There is evidence that ACE inhibition by ramipril appears to have some effects on the kallikrein-kinin-prostaglandin systems. It is assumed that effects on these systems contribute to the hypotensive and metabolic activity of ramipril.
Administration of Triatec to hypertensive patients results in reduction of both supine and standing blood pressure. The antihypertensive effect is evidence within 1-2 hours after the drug intake: peak effect occurs 3-6 hours after drug intake and has been shown to be maintained for at least 24 hours after usual therapeutic doses.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
