SOMEZOL Capsule
ក្រុមហ៊ុនផលិតឱសថ:
Bosch Pharmaceuticals (PVT) Ltd, Pakistan
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
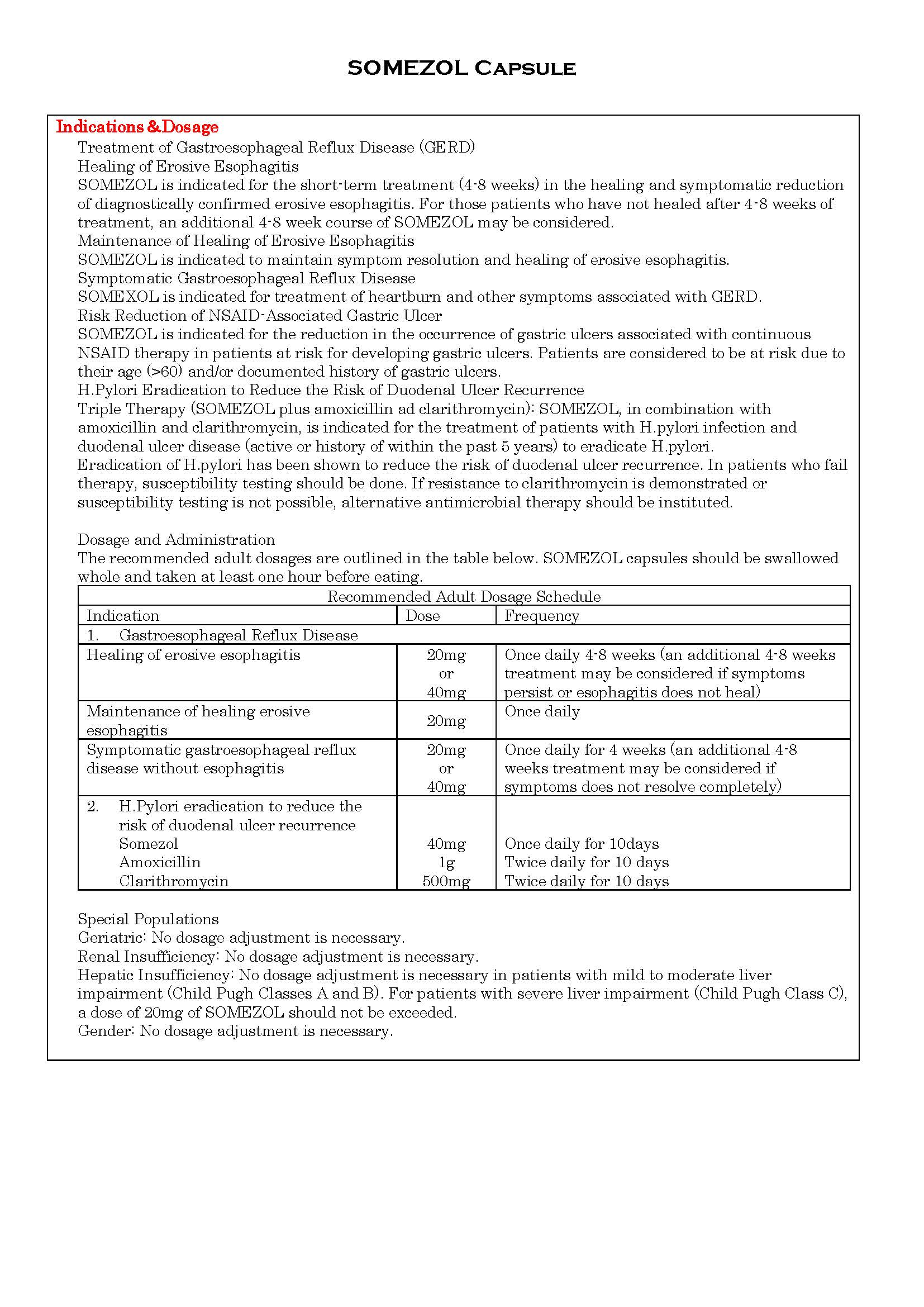
-
ហាមប្រើ
In patients with known hypersensitivity to any component of the formulation or to substituted benzimidazoles.
-
ផលរំខាន
None found to be dose-related
Common: Headache, abdominal pain, diarrhoea, flatulence, nausea/vomiting, constipation
Uncommon: Dermatitis, pruritis, urticaria, dizziness, dry mouth
Rare: Hypersensitivity reactions e.g. angioedema, anaphylactic reaction
-
អន្តរប្រតិកម្ម
Drug interaction studies have shown that Esomeprazole does not have any clinically significant interactions with phenytoin, warfarin, quinidine, clarithromycin or amoxicillin. Coadministration of oral contraceptives, diazepam, phenytoin, or quinidine did not seem to change the pharmacokinetic profile of Esomeprazole. Esomeprazole inhibits gastric acid secretion. Therefore, Esomeprazole may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (e.g. ketoconazole, iron salts and digoxin).
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
Teratogenic Effects: Pregnancy Category B- Teratology studies have been performed in rats at oral doses up to 280mg/kg/day (about 57 times the human dose on a body surface area basis) and in rabbits at oral doses up to 86mg/kg/day (about 35 times the human dose on a body surface area basis) and have revealed no evidence of impaired fertility or harm to the fetus due to Esomeprazole. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
The excretion of Esomeprazole in milk has not been studied. However, omeprazole concentrations have been measured in breast milk of a woman following oral administration of 20mg. Because Esomeprazole is likely to be excreted in human milk. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ការប្រុងប្រយ័ត្នជាពិសេស
General
In the presence of any alarming symptoms (e.g. significant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with Esomeprazole may alleviate symptoms and delay diagnosis. Patients on long term treatment (particularly those treated for more than a year) should be kept under regular surveillance since the symptomatic response to therapy with Esomeprazole does not preclude the gastric malignancy.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
No overall differences is safety and efficacy sere observed between the elderly and younger individuals, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
