RIBAZOLE Capsule
ក្រុមហ៊ុនផលិតឱសថ:
Getz pharma, USA
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
ALLIANCE PHARMA CAMBODGE
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Ribavirin 400mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
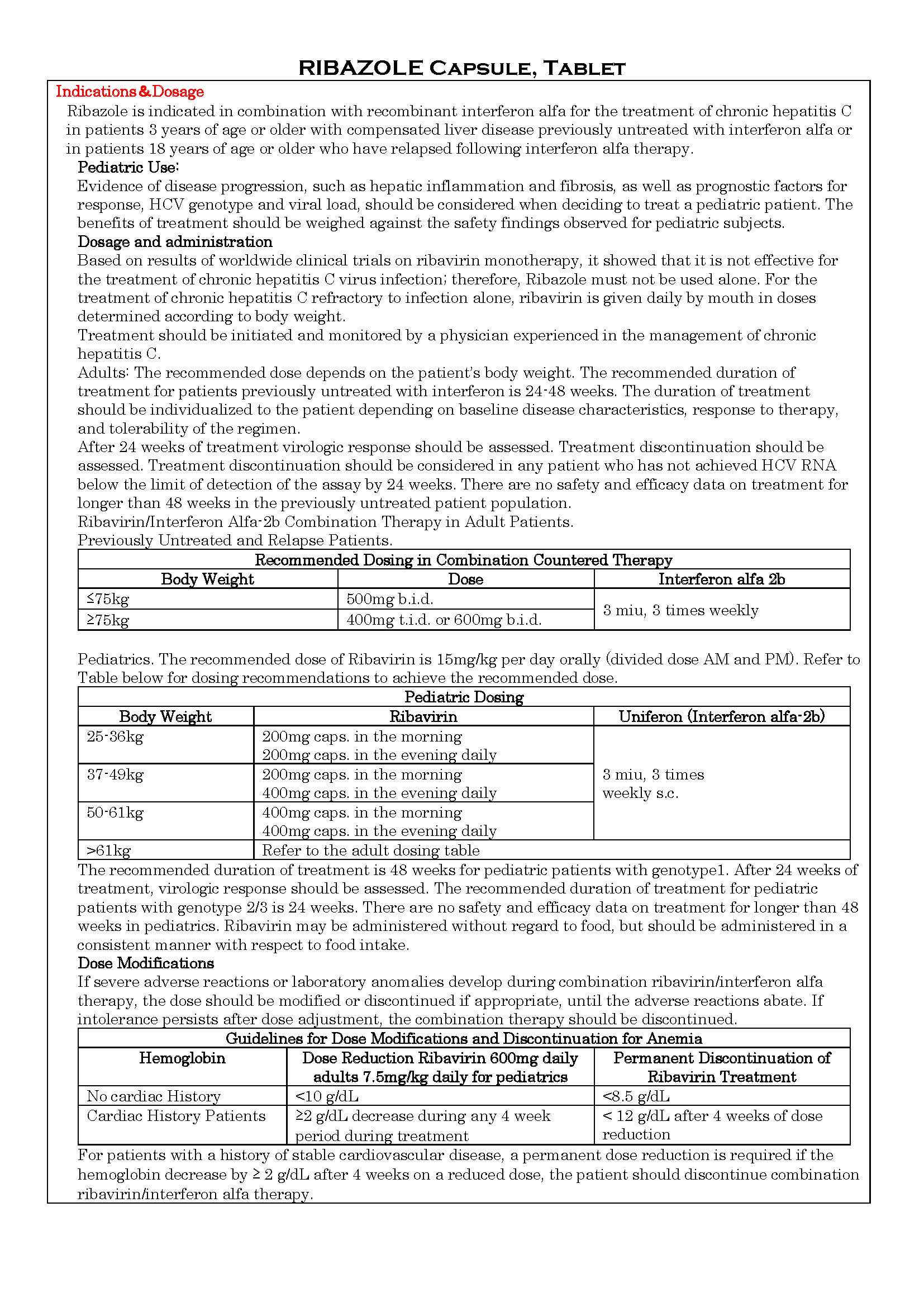
-
ហាមប្រើ
- Patients with a history of known hypersensitivity to ribavirin or any of its components.
- Ribavirin therapy is contraindicated for use in pregnant women or in women expected to be pregnant.
- Patients with autoimmune hepatitis must not be treated with combination of ribavirin and interferon alfa therapy because use of these medicines can worsen the hepatitis.
- Patients with hemoglobinopathies (e.g. thalassemia major, sickle-cell anemia) should not be treated with ribavirin.
- Patients with a history of severe pre-existing cardiac disease, including unstable or uncontrolled cardiac disease.
-
ផលរំខាន
The primary toxicity of ribavirin is hemolytic anemia. Patients receiving ribavirin by mouth have experienced hemolytic anemia, sometimes with associated increased serum concentrations of bilirubin and uric acid. The most commonly reported adverse reactions with ribavirin were mostly mild to moderate in severity and were manageable without the need for modification of doses or discontinuation of therapy. They include depression, irritability, anxiety, alopecia, nausea/vomiting, and flu like symptoms such as fatigue, pyrexia, myalgia, headache and rigors.
Ribavirin/Interferon alfa Combination Therapy
There are significant adverse events caused by ribavirin/interferon alfa combination therapy, which includes severe depression and suicidal ideation, hemolytic anemia, suppression of bone marrow function, autoimmune and infectious disorders, pulmonary dysfunction, pancreatitis, and diabetes. It is recommended that patients be carefully monitored by the prescribing physician.
Other less frequent adverse reactions were pruritis, dermatitis, dizziness, arthralgia, neutropenia, insomnia, diarrhoea, dyspnea, dry skin and skin rashes.
Pediatric patients
Conversely, pediatric patients experienced less fatigue, dyspepsia, arthralgia, insomnia, irritability, impaired concentration, dyspnea, and pruritis compared to adult patients.
-
អន្តរប្រតិកម្ម
There is no clinically relevant interaction when ribavirin is concomitantly administered with antacids.
Nucleoside analogues
See the package insert about the details below:
- Zidovudine and Stavudine
- Didanosine
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
Extreme care must be taken to avoid pregnancy in female patients and in partners of male patients. Ribavirin accumulates intracellularly and is cleared from the body very slowly. Ribavirin therapy must not be initiated until a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. Any birth control method can fail. Therefore, it is critically important that women of childbearing potential and their partners must use 2 forms of effective contraception simultaneously, during treatment and for 6 months after treatment has been concluded. Routine monthly pregnancy tests must be performed during this time.
Lactation
It is not known whether ribavirin is excreted in human milk. Because of the potential for adverse reactions in nursing infants, nursing must be discontinued prior to initiation of treatment.
-
ការប្រុងប្រយ័ត្នជាពិសេស
See the package insert about the details below:
- Haemolysis and cardiovascular system
- Liver function
- Psychiatric and Central Nervous System (CNS)
- Renal impairment
-
សកម្មភាពឱសថ
Ribavirin is a synthetic guanine analogue that acts in a manner similar to the other nucleoside analogues. It shows in vitro activity against some RNA and DNA viruses. RNA is essential in the synthesis of proteins, as the messenger with the genetic code of RNA and DNA viruses. Ribavirin blocks messenger RNA, prevents viral replication and stops the infection. Ribavirin suppresses viral replication without effect on normal cellular function.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
