ORLIVEX Capsule
ក្រុមហ៊ុនផលិតឱសថ:
VEXXA IFESCIENCES PVT.LTD., India
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
1. ORLIVEX 60:
Orlistat 60mg
2. ORLIVEX 120:
Orlistat 120mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
In conjunction with a mildly hypocaloric diet for the treatment of obese patients with a body mass index (BMI) greater or equal to 30kg/m2, or overweight patients (BMI≥28kg/m2) with associated risk factors.
Treatment with Orlistat should be discontinued after 12 weeks if patients have been unable to lose at least 5% of the body weight as measured at the start of therapy.
Posology and method of administration
Adults
The recommended dose is 120mg taken with water immediately before, during or up to 1 hour after each main meal. If a meal is missed or contains no fat, the dose should be omitted.
The patient should be on a nutritionally balanced, mildly hypocaloric diet that contains approximately 30% of calories from fat. It is recommended that the diet should be rich in fruit and vegetables. The daily intake of fat, carbohydrate and protein should be distributed over 3 main meals.
Doses above 120mg 3 times daily have not been shown to provide additional benefit.
The effect of Orlistat results in an increase in faecal fat as early as 24-48 hours after dosing. Upon discontinuation of therapy faecal fat content usually returns to pre-treatment levels, within 48-72 hours.
Special populations
The effect in patients with hepatic and/or renal impairment, children and elderly patients has not been studied.
There is no relevant indication for use of Orlistat Capsules in children.
-
ហាមប្រើ
- Hypersensitivity to the active substance or to any of the excipients.
- Chronic malabsorption syndrome.
- Cholestasis.
- Breast-feeding.
-
ផលរំខាន
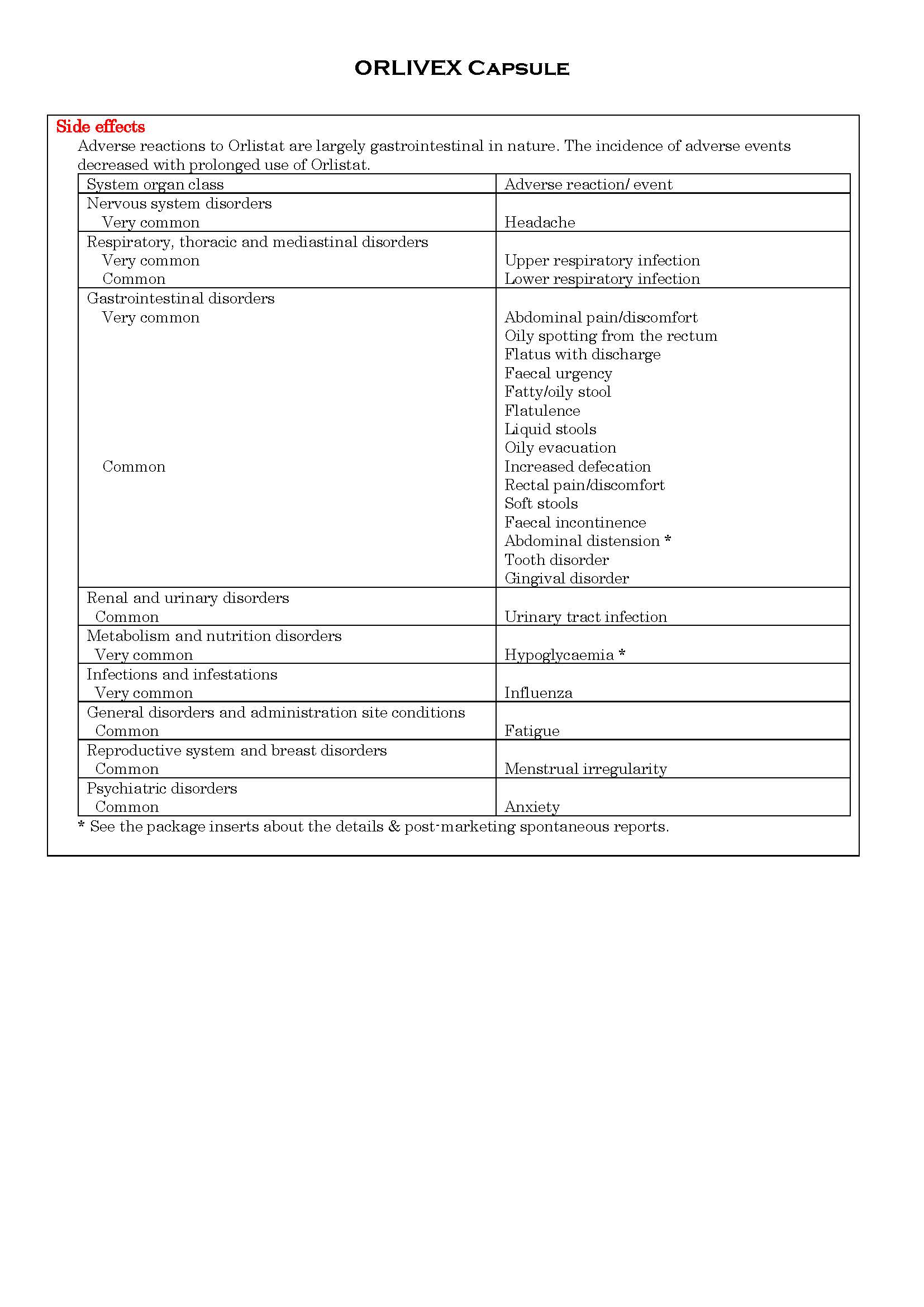
-
អន្តរប្រតិកម្ម
See the package insert about the details below:
Ciclosporin
Acarbose
Oral anticoagulants
Fat soluble vitamins
Amiodarone
Antiepileptic drugs e.g. valproate, lamotrigine
Rare occurrence of hypothyroidism and/or reduced control of hypothyroidism may occur. The mechanism, although not proven, may involve a decreased absorption of iodine salts and/or levothyroxine.
Lack of interactions: No interactions with amitriptyline, atorvastatin, biguanides, digoxin, fibrates, fluoxetine, losartan, phenytoin, phentermine, pravastatin, nifedipine, Gastrointestinal Therapeutic System (GITS), nifedipine slow release, sibutramine or alcohol have been observed. The absence of these interactions has been demonstrated in specific drug-drug-interaction studies.
The absence of an interaction between oral contraceptives and Orlistat has been demonstrated in specific drug-drug interaction studies. However, Orlistat may indirectly reduce the availability of oral contraceptives and lead to unexpected pregnancies in some individual cases. An additional contraceptive method is recommended in case of severe diarrhoea.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
Lactation
Contraindicated.
-
ការប្រុងប្រយ័ត្នជាពិសេស
In clinical trials, the decrease in bodyweight with Orlistat treatment was less in typeⅡdiabetic patients than in non-diabetic patients.
Antidiabetic medicinal product treatment may have to be closely monitored when taking Orlistat.
Co-administration of Orlistat with ciclosporin is not recommended.
Patients should be advised to adhere to the dietary recommendation they are given.
The possibility of experiencing gastrointestinal adverse reactions may increase when Orlistat is taken with a diet high in fat (e.g. in a 2000kcal/day diet, >30% of calories from fat equates to >67g of fat). The daily intake of fat should be distributed over 3 main meals. If Orlistat is taken with a meal very high in fat, the possibility of gastrointestinal adverse reactions may increase.
Cases of rectal bleeding have been reported with Orlistat Capsules. Prescribers should investigate further in case of severe and/or persistent symptoms.
The use of an additional contraceptive method is recommended to prevent possible failure of oral contraception that could occur in case of severe diarrhoea.
Coagulation parameters should be monitored in patients treated with concomitant oral anticoagulants.
The use of Orlistat may be associated with hyperoxaluria and oxalate nephropathy in patients with underlying chronic kidney disease and/or volume depletion.
Rare occurrence of hypothyroidism and/or reduced control of hypothyroidism may occur. The mechanism, although not proven, may involve a decreased absorption of iodine salts and/or levothyroxine.
Antiepileptics patient: Orlistat may unbalance anticonvulsivant treatment by decreasing the absorption of antiepileptic drugs, leading to convulsions.
-
សកម្មភាពឱសថ
Pharmaco-therapeutic group: Peripherally acting antiobesity agent.
Orlistat is a potent, specific and long-acting inhibitor of gastrointestinal lipases. It exerts its therapeutic activity in the lumen of the stomach and small intestine by forming a covalent bond with the active serine site of the gastric and pancreatic lipases. The inactivated enzyme is thus unavailable to hydrolyse dietary fat, in the form of triglycerides, into absorbable free fatty acids and monoglycerides.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
