METHYLBOSTON 16 Tablet
ក្រុមហ៊ុនផលិតឱសថ:
BOSTON VIETNAM PHARMACEUTICAL JOINT STOCK COMPANY, Vietnam
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Methylprednisolone 16mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
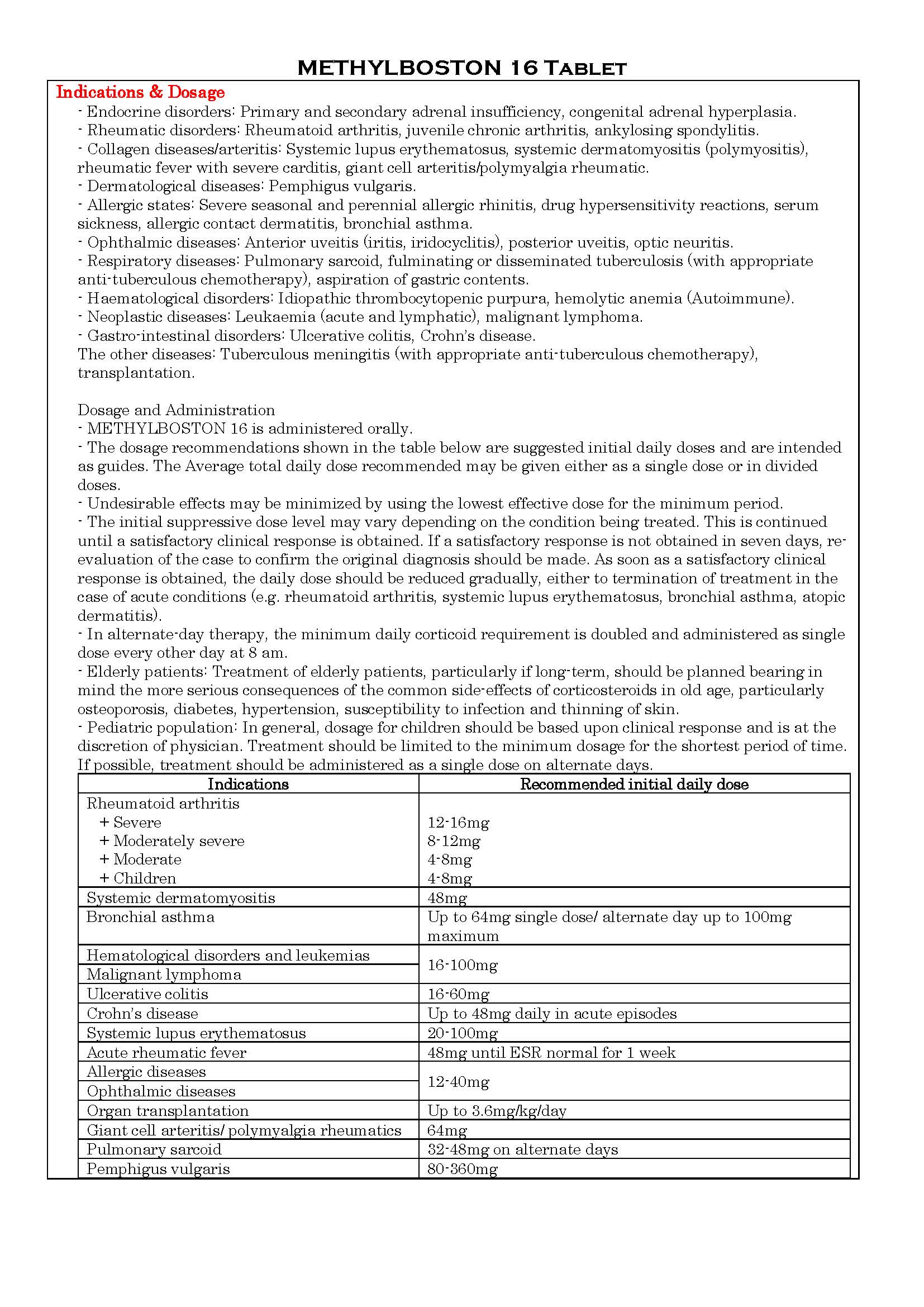
-
ហាមប្រើ
- Patients with systemic fungal infections.
- Patients with systemic infection unless specific anti-infective therapy is employed.
- Patients who have hypersensitivity to methylprednisolone or to any of the excipients.
- Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids.
-
ផលរំខាន
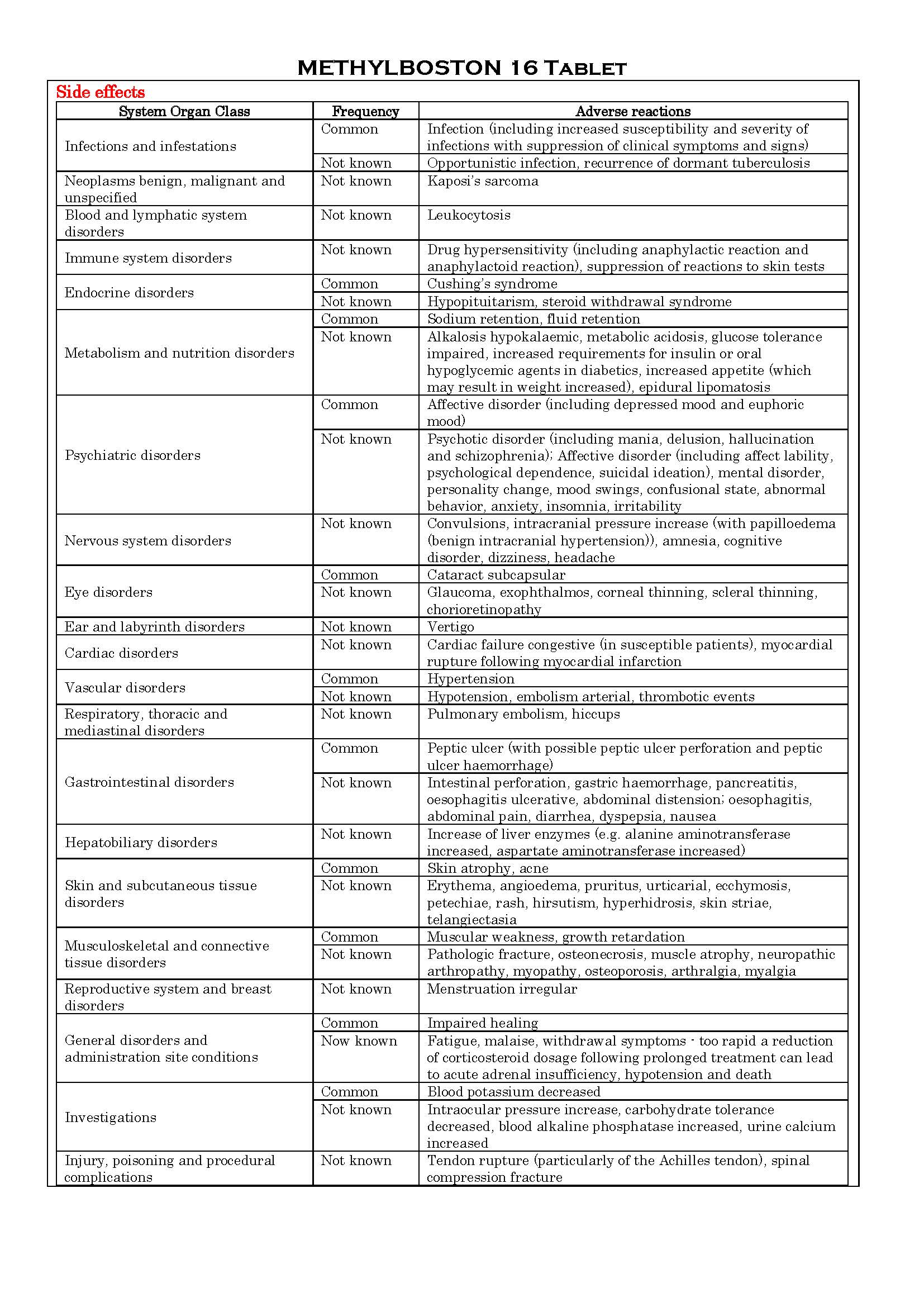
-
អន្តរប្រតិកម្ម
See the package insert about the details below:
- CYP3A4 inducers (Anticonvulsants such as phenobarbital, phenytoin, carbamazepine and primidone, Bactericidal antibiotics such as rifampicin and rifabutin).
- CYP3A4 inhibitors (Antifungal such as ketoconazole and itraconazole, Antiemetics such aprepitant and fosaprepitant, Immunosuppressant such as ciclosporin, Macrolide antibacterials such as clarithromycin ,erythromycin and troleandomycin, HIV-protease inhibitors such indinavir and ritonavir, Calcium channel blockers such as diltiazem, Isoniazid, Oral contraceptives such as ethinylestradiol and norethisterone, Grapefruit juice).
- CYP3A4 substrates (Immunosuppressants such as cyclophosphamide ad tacrolimus).
- Other interactions (Antidiabetic agents, Anticholinergics, Anticholinesterases, Anticoagulants (oral), Aromatase inhibitors, Cardiac glycosides, Diuretics and other potassium depleting agents, NSAIDs, Vaccines).
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
See the package insert about the details below:
Pregnancy
There is no evidence that corticosteroids result in an increased incidence of congenital abnormalities, such as cleft palate in man, however, when administered for long periods or repeatedly during pregnancy, corticosteroids may increase the risk of intra-uterine growth retardation.
Cataracts have been observed in infants born to mothers undergoing long-term treatment with corticosteroid during pregnancy.
Lactation
Since adequate reproductive studies have not been performed in humans with glucocorticoids, these drugs should be administered to nursing mothers only if the benefits of therapy are judged to outweigh the potential risks to the infant.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Immunosuppressant effects/Increased susceptibility to infections
- Corticosteroid may increase susceptibility to infection, may mask some signs of infection, and new infections may appear during their use. Suppression of the inflammatory response and immune function increases the susceptibility to fungal, viral and bacterial infections and their severity. The clinical presentation may often be atypical and may reach an advanced stage before being recognized. Chicken pox and measles, for example, can have a more serious or even fatal course in non-immune children or adults on corticosteroids.
- Patients (or patients of children) without a definite history of chickenpox should be advised to avoid close personal contact with chickenpox or herpes zoster and if exposed they should seek urgent medical attention.
- Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. The antibody response to other vaccines may be diminished.
- The use of corticosteroids in active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate antituberculosis regimen. If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
Immune system
- Because rare instances of skin reactions and anaphylactic/anaphylactoid reactions have occurred in patients receiving corticosteroid therapy, appropriate precautionary measures should be taken prior to administration, especially when the patient has a history of allergy to any drug.
Endocrine effects
- In patients on corticosteroid therapy subjected to unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is indicated.
- Adrenal cortical atrophy develops during prolonged therapy and may persist for months after stopping treatment. In patients who have received more than physiological doses of systemic corticosteroids (approximately 6mg methylprednisolone) for greater than 3 weeks, withdrawal should not be abrupt. How dose reduction should be carried out depends largely on whether the disease is likely to relapse as the dose of systemic corticosteroids is reduced.
- If the disease is unlikely to relapse on withdrawal of systemic corticosteroids, but there is uncertainty about HPA suppression, the dose of systemic corticosteroid may be reduced rapidly to physiological doses.
- Abrupt withdrawal of doses up to 32mg daily of methylprednisolone for 3 weeks is unlikely to lead to clinically relevant HPA suppression, in the majority of patients. In the following patient groups, gradual withdrawal of systemic corticosteroid therapy should be considered even after courses lasting 3 weeks or less:
+ Patients who have had repeated courses of systemic corticosteroids, particularly if taken for greater than 3 weeks.
+ When a short course has been prescribed within 1 year of cessation of long-term therapy (months or years).
+ Patients who may have reasons for adrenocortical insufficiency other than exogenous corticosteroid therapy. In addition, acute adrenal insufficiency leading to a fatal outcome may occur if glucocorticoids are withdrawn abruptly.
+ Patients receiving doses of systemic corticosteroid greater than 32mg daily of methylprednisolone.
+ Patients repeatedly taking doses in the evening.
- Glucocorticoids can produce or aggravate Cushing’s syndrome, therefore glucocorticoids should be avoided in patients with Cushing’s disease.
Particular care is required when considering the use of systemic corticosteroids in patients with hypothyroidism and frequent patient monitoring is necessary.
Metabolism and nutrition disorders
- Corticosteroids, including methylprednisolone, can increase blood glucose, worsen pre-existing diabetes, and predispose those on long-term corticosteroid therapy to diabetes mellitus. Particular care is required when considering the use of systemic corticosteroids in patients with diabetes mellitus (or a family history of diabetes).
Psychiatric effects
- Patients and/or carers should be warned that potentially severe psychiatric adverse reactions may occur with systemic steroids. Symptoms typically emerge within a few days or weeks of starting treatment. Most reactions recover after either dose reduction or withdrawal, although specific treatment may be necessary.
Patients/carers should be alert to possible psychiatric disturbances that may occur either during or immediately after dose tapering/withdrawal of systemic steroids, although such reactions have been reported infrequently.
- Particular care is required when considering the use of systemic corticosteroids in patients with existing or previous history of severe affective disorders in themselves or in their first degree relatives.
Nervous effects
- Caution for patients with seizure disorders and myasthenia gravis.
Ocular effects
- Caution for patients with glaucoma (or a family history of glaucoma) and ocular herpes simplexas there is fear of conceal perforation. Prolonged use of corticosteroids may produce posterior subcapsular cataracts and nuclear cataracts (particularly in children), exophthalmos or increased intraocular pressure, which may result in glaucoma with possible damage to the optic nerves. Secondary fungal and viral infections of the eyes may also enhanced in patients receiving glucocorticoids. Corticosteroids therapy has been associated with chorioretinopathy, which may lead to retinal detachment.
Cardiac events and vascular effects
- Adverse effects of glucocorticoids on the cardiovascular system, such as dyslipidemia and hypertension, may predispose treated patients with existing cardiovascular risk factors to additional cardiovascular effects, if high doses and prolonged courses are used. Accordingly, corticosteroids additional cardiac monitoring if needed. Low dose and alternate day therapy may reduce the incidence of complications in corticosteroid therapy.
- Systemic corticosteroid should be used with caution and only if strictly necessary, in cases of congestive heart failure.
- Care should be taken for patients receiving cardioactive drugs such as digoxin.
Particular care is required when considering the use of systemic corticosteroids in patients with recent myocardial infarction, patients with hypertension, patients with predisposition to thrombophlebitis and patients who have thromboembolic disorders.
Gastrointestinal effects
- Caution for patients with peptic ulceration, fresh intestinal anastomoses, abscess or other pyogenic infections, ulcerative colitis, diverticulitis. In combination with NSAIDs, the risk of developing gastrointestinal ulcers is increased.
Hepatobiliary effects
- High doses of corticosteroids may produce acute pancreatitis. Caution for patients with liver failure or cirrhosis.
Musculoskeletal effects
- An acute myopathy has been reported with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (myasthenia gravis) r in patients receiving concomitant therapy with anticholinergics, such as neuromuscular blocking drugs (pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevations of creatine kinase may occur. Particular care is required when considering the use of systemic corticosteroids in patients with osteoporosis (post-menopausal females are particularly at risk) and frequent patient monitoring is necessary.
Renal and urinary
- Particular care is required when considering the use of systemic corticosteroids in patients with renal insufficiency and frequent patient monitoring is necessary.
Pediatric population
- Corticosteroids cause growth retardation in infancy, childhood and adolescence. Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed.
- Infants and children on prolonged corticosteroid therapy are at special risk from raised intracranial pressure.
- High doses of corticosteroids may produce pancreatitis in children.
Others
- This medicine contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
This product contains erythrosine. It may cause allergic reactions.
-
សកម្មភាពឱសថ
Glucocorticosteroids
Methylprednisolone is a synthetic glucocorticoid and a methyl derivative of prednisolone. Methylprednisolone is a potent anti-inflammatory agent with the capacity to profoundly inhibit the immune system.
See the package insert about the details below:
- Intracellular action of glucocorticoids
- Carbohydrate, protein and lipid metabolism
- Effects on muscle, bone and connective tissue
- Effects on the vascular system
- Effects on the central nervous system
- Effects on inflammatory and immune responses.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
