MAXPRO Tablet
ក្រុមហ៊ុនផលិតឱសថ:
RENATA LIMITED, Bangladesh
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
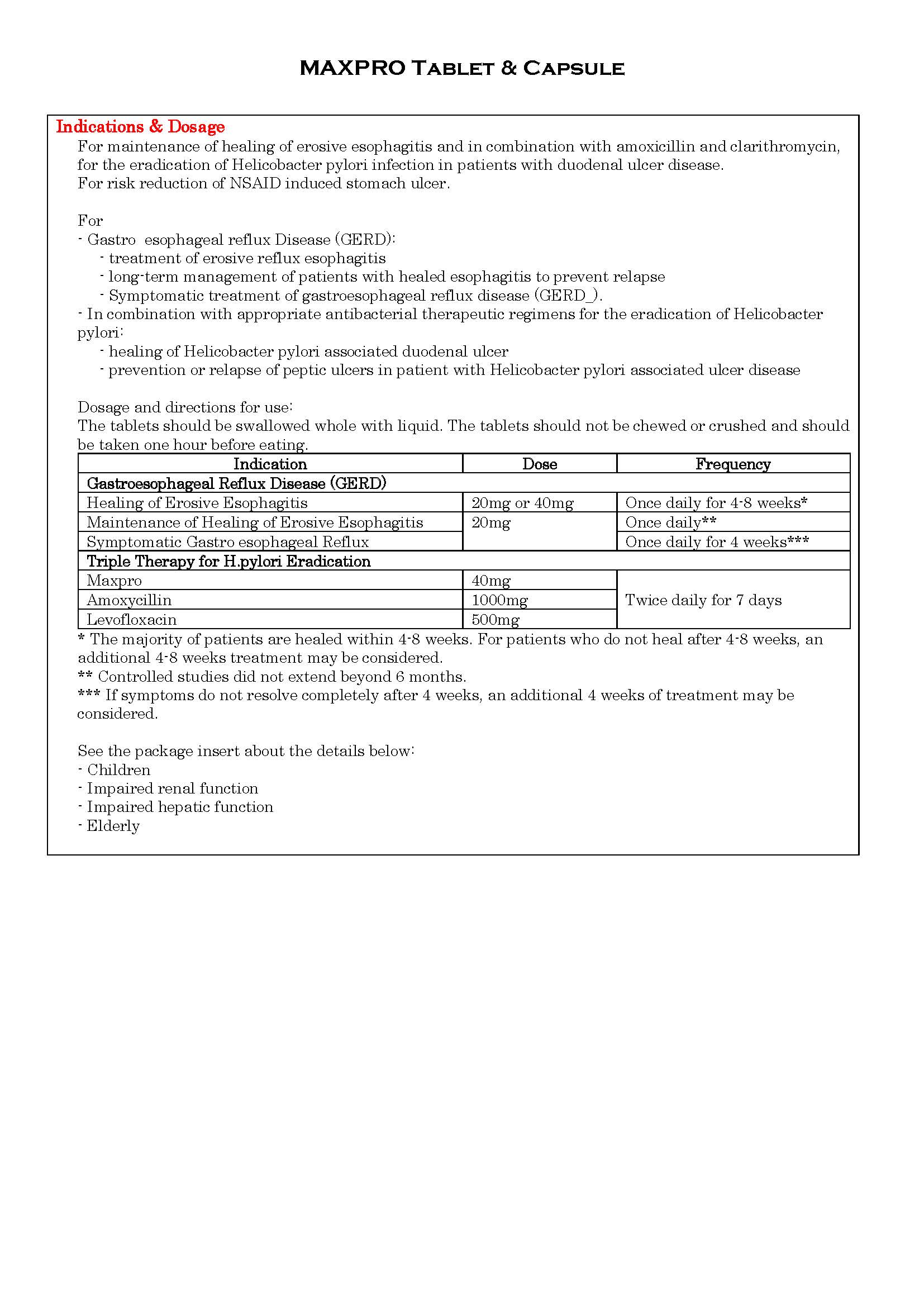
-
ហាមប្រើ
Known hypersensitivity to esomeprazole, substituted benzimidazoles or any other constituents of the formulation.
-
ផលរំខាន
More frequent: Headache, abdominal pain, diarrhoea, flatulence, nausea/vomiting,
Less frequent: Dermatitis, pruritus, urticaria, dizziness, mouth.
-
អន្តរប្រតិកម្ម
In common with the use of other inhibitors of acid secretion or antacids, the absorption of ketoconazole and itraconazole can decrease during treatment with esomeprazole.
When esomeprazole is combined with drugs, such as diazepam, citalopram, imipramine, clomipramine, phenytoin etc, the plasma concentrations of these drugs may be increased and a dose reduction could be needed.
Concomitant administration of 30mg esomeprazole resulted in a 45% decrease in clearance of the CYP2C19 substrate diazepam.
Concomitant administration of 40mg esomeprazole resulted in a 13% increase in trough plasma levels of phenytoin in epileptic patients.
It is recommended to monitor the plasma concentrations of phenytoin when treatment with esomeprazole is introduced or withdrawn.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
FDA pregnancy category B. No fatal teratogenicity or harm. Limited human pregnancy data. Use is acceptable for aspiration prophylaxis during pregnancy.
-
ការប្រុងប្រយ័ត្នជាពិសេស
In the presence of any alarm symptom (e.g. significant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with esomeprazole may alleviate symptoms and delay diagnosis.
Patient on long-term treatment (particularly those treated for more than a year), should be kept under regular surveillance.
-
សកម្មភាពឱសថ
Esomeprazole, the S-isomer of omeprazole, reduces gastric acid secretion through specific inhibition of the acid pump in the parietal cell, where it is concentrated and converted to the active form in the acid environment of the secretary canaliculi and inhibits the enzyme H+K+ATPase - the acid pump. This effect on the final step of the gastric acid secretion is dose-dependent and provides for effective inhibition of both basal and stimulated acid secretion. Food intake had no significant influence on the effect of esomeprazole on intragastric acidity.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
