LOPID Tablet
ក្រុមហ៊ុនផលិតឱសថ:
OLIC (Thailand) Limited, Thailand
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
DKSH


- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Gemfibrozil 600mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
Gemfibrozil is a liquid regulating agent that is indicated for the following:
1. Primary prevention of coronary heart disease (CHD) and myocardial infarction (MI) in patients with hypercholesterolemia, mixed dyslipidemia and hypertriglyceridemia, Fredrickson’s classification types Ⅱa, Ⅱb and Ⅳ.
2. Treatment of other dyslipidemias:
a) Fredrickson types Ⅲ and Ⅳ
b) Associated with diabetes
c) Associated with xanthomata
3. Treatment of adult patients with elevated levels of serum triglycerides (types Ⅳand Ⅴ hyperlipidemia) who present a risk of pancreatitis and do not respond adequately to a determined dietary effort to control them.
Posology and method of administration
General
Lipid levels should be measured on more than one occasion, to ascertain that the levels are consistently abnormal. Before instituting therapy with gemfibrozil, every attempt should be made to control serum lipids with appropriate diet, limiting alcohol intake, exercise, and weight loss in obese patients, as well as controlling other medical problems such as diabetes mellitus of hypothyroidism, which may contribute to the abnormal lipid levels. The patient should continue a standard cholesterol-lowering diet during treatment with gemfibrozil. Periodic determinations of serum lipids should be obtained during treatment with gemfibrozil. The drug should be withdrawn or additional therapy instituted if the lipid response is inadequate after 3 months.
The recommended daily dose is 900mg-1200mg. The maximum daily dose is 1500mg. The 900mg dose is given as a single dose one-half hour before the evening meal. The 1200mg dose is given in two divided doses one-half hour before the morning and evening meals.
Use in Patients with Hepatic/Renal Dysfunction, - See sections “Contraindications” and “Special Precaution”
Use in Children - Safety and efficacy in children have not been established.
-
ហាមប្រើ
Gemfibrozil is contraindicated in patients with hepatic or severe renal dysfunction, pre-existing gallbladder disease, and in patients who are hypersensitive to gemfibrozil or any of the inert ingredients.
The concomitant use of gemfibrozil is contraindicated with any of the following:
- simvastatin
- repaglinide
- basabuvir
-
ផលរំខាន
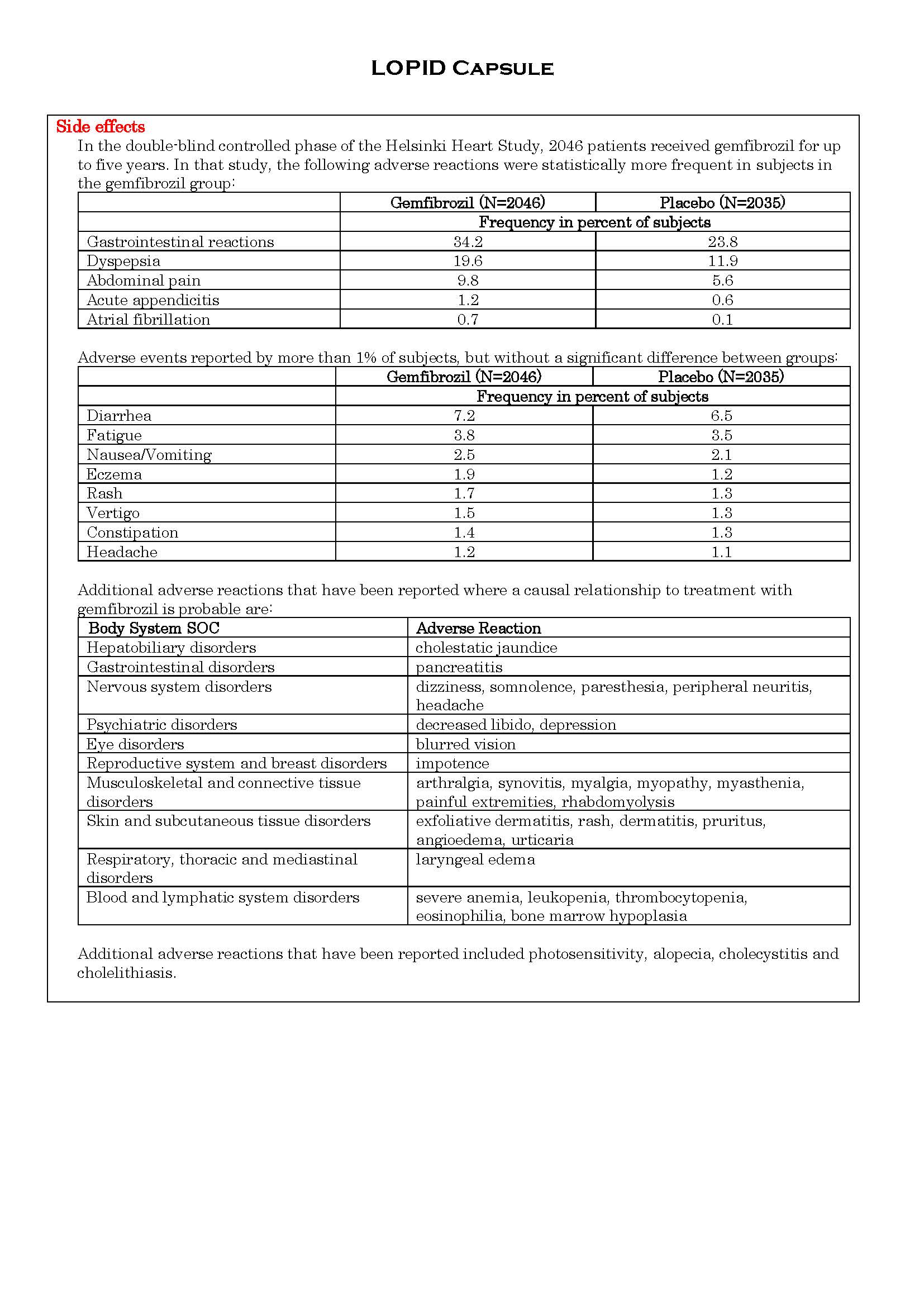
-
អន្តរប្រតិកម្ម
Anticoagulants
Caution should be exercised when warfarin is given in conjunction with gemfibrozil. The dosage of warfarin should be reduced to maintain the prothrombin time at the desired level to prevent bleeding complications. Frequent prothrombin time determinations are advisable until it has been definitely determined that the prothrombin time has stabilized.
HMG-CoA Reductase Inhibitors
The concomitant administration of gemfibrozil with simvastatin is contraindicated. There have been reports of severe myositis and myoglobinuria (rhabdomyolysis) when gemfibrozil and HMG-CoA reductase inhibitors were used concomitantly.
CYP2C8 substrates
Gemfibrozil is an inhibitor of CYP2C8 and may increase exposure of drugs mainly metabolized by CYP2C8 (e.g., dabrafenib, loperamide, montelukast, paclitaxel, pioglitazone, rosiglitazone). Therefore, dosing reduction of drugs that are mainly metabolized by CYP2C8 enzyme may be required when gemfibrozil is used concomitantly.
Co-administration of gemfibrozil and repaglinide increases the risk for severe hypoglycemia and is contraindicated.
Itraconazole, basabuvir (see the package insert).
Bile Acid-binding Resins
Reduced bioavailability of gemfibrozil may result when given simultaneously with resin-granule drugs such as colestipol. Administration of the drugs 2 hours apart or more is recommended.
Colchicine
Risk of neuromuscular toxicity and rhabdomyolysis may be increased with concomitant administration of colchicine and gemfibrozil. This risk may be increased in the elderly and in patients with hepatic or renal dysfunction. Symptoms usually last between 1 week and several months after colchicine withdrawal. Clinical a biological monitoring is recommended, especially at the start of combined treatment.
In-vitro studies of CYP enzymes, UGTA enzymes and OATP1B1 transporter
In-vitro studies have shown that gemfibrozil is an inhibitor of CYP1A2, CYP2C8, CYP2C9, CYP2C19, organic anion-transporting polypeptide (OATP)1B1 and UDP-glucuronosyltransferase (UGT)1A1 and 1A3.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
There are no adequate ad well-controlled studies in pregnant women. The use of gemfibrozil in pregnancy should be reserved for those patients where the benefits clearly outweigh the risks to the patient or fetus.
Safety in nursing mothers has not been established. It is not known whether gemfibrozil is excreted in human milk. Since many drugs are excreted in human milk, the patient should discontinue nursing before beginning gemfibrozil therapy.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Cholelithiasis
Gemfibrozil may increase cholesterol excretion into the bile, raising the potential for gallstone formation. If cholelithiasis is suspected, gallbladder studies are indicated. Gemfibrozil therapy should be discontinued if gallstones are found. Cases of cholelithiasis have been reported with gemfibrozil therapy.
HMG-CoA Reductase Inhibitors
The concomitant administration of gemfibrozil with simvastatin is contraindicated. There have been reports of severe myositis with markedly elevated creatine kinase (CK) and myoglobinuria (rhabdomyolysis) when gemfibrozil and HMG-CoA reductase inhibitors were used concomitantly. In most subjects who have had an unsatisfactory lipid response to either drug alone, the possible benefit of combined therapy with HMG-CoA reductase inhibitors and gemfibrozil does not outweigh the risks of severe myopathy, rhabdomyolysis, and acute renal failure.
Anticoagulants
Caution should be exercised with concomitant use of warfarin. The dosage of warfarin should be reduced to maintain the prothrombin time at the desired level to prevent bleeding complications. Frequent prothrombin time determinations are advisable until it has been definitely determined that the prothrombin time has stabilized.
CYP2C8 substrates
Gemfibrozil, an inhibitor of CYP2C8, may increase exposure of CYP2C8 substrates when administered concomitantly.
Laboratory Tests
Elevated liver function tests(LFTs) such as liver transaminases (AST, sGOT, and ALT, sGPT), increased alkaline phosphatase, LDH, CK, and bilirubin have rarely been reported with gemfibrozil administration. These are usually reversible when gemfibrozil is discontinued. Therefore, periodic liver function studies are recommended, and gemfibrozil therapy should be terminated if abnormalities persist.
Hematopoietic
Mild decreases in hemoglobin, hematocrit and white cell have been observed occasionally on initiating gemfibrozil therapy. However, these levels stabilize during long-term administration. Rarely, severe anemia, leukopenia, thrombocytopenia, eosinophilia and bone marrow hypoplasia have been reported. Therefore, periodic blood count determinations are recommended during the first 12 months of gemfibrozil administration.
-
សកម្មភាពឱសថ
Gemfibrozil’s mechanism of action has not been definitively established. In humans, gemfibrozil inhibits peripheral lipolysis and decreases the hepatic extraction of free fatty acids. Gemfibrozil also inhibits synthesis and increases clearance of apolipoprotein B, which is a carrier of very-low-density lipoprotein(VLDL), leading to a decrease in VLDL production. Gemfibrozil increases the level of high density lipoprotein(HDL) subfractions, HDL2 and HDL3, as well as apolipoprotein A-1 and A-2. Animal studies suggest that the turnover and removal of cholesterol from the liver is increased by gemfibrozil.
Gemfibrozil is a lipid-regulating agent, which reduces total cholesterol, low-density lipoprotein(LDL) cholesterol, VLDL and triglycerides and increases HDL cholesterol.
In the Helsinki Heart Study, a large, randomized, double-blind, placebo-controlled primary prevention trial, involving subjects with serum non-HDL cholesterol over 200mg/mL and no previous history of heart disease, gemfibrozil produced a significant reduction in total plasma triglycerides, moderate reductions in total and LDL cholesterol and a significant increase in HDL cholesterol. Over the 5-year study period, the gemfibrozil group experienced a 34% reduction in the overall incidence of CHD (in years 4 and 5 of the study, the reduction in CHD was greater than 50%). There was a 37% reduction in non-fatal MI and a 26% reduction in cardiac deaths. The overall difference in the incidence of CHD was significantly lower for gemfibrozil-treated patients than for those receiving placebo (p<0.02, two-tailed).
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
