EZIUM Capsule
ក្រុមហ៊ុនផលិតឱសថ:
SEARLE, Pakistan
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
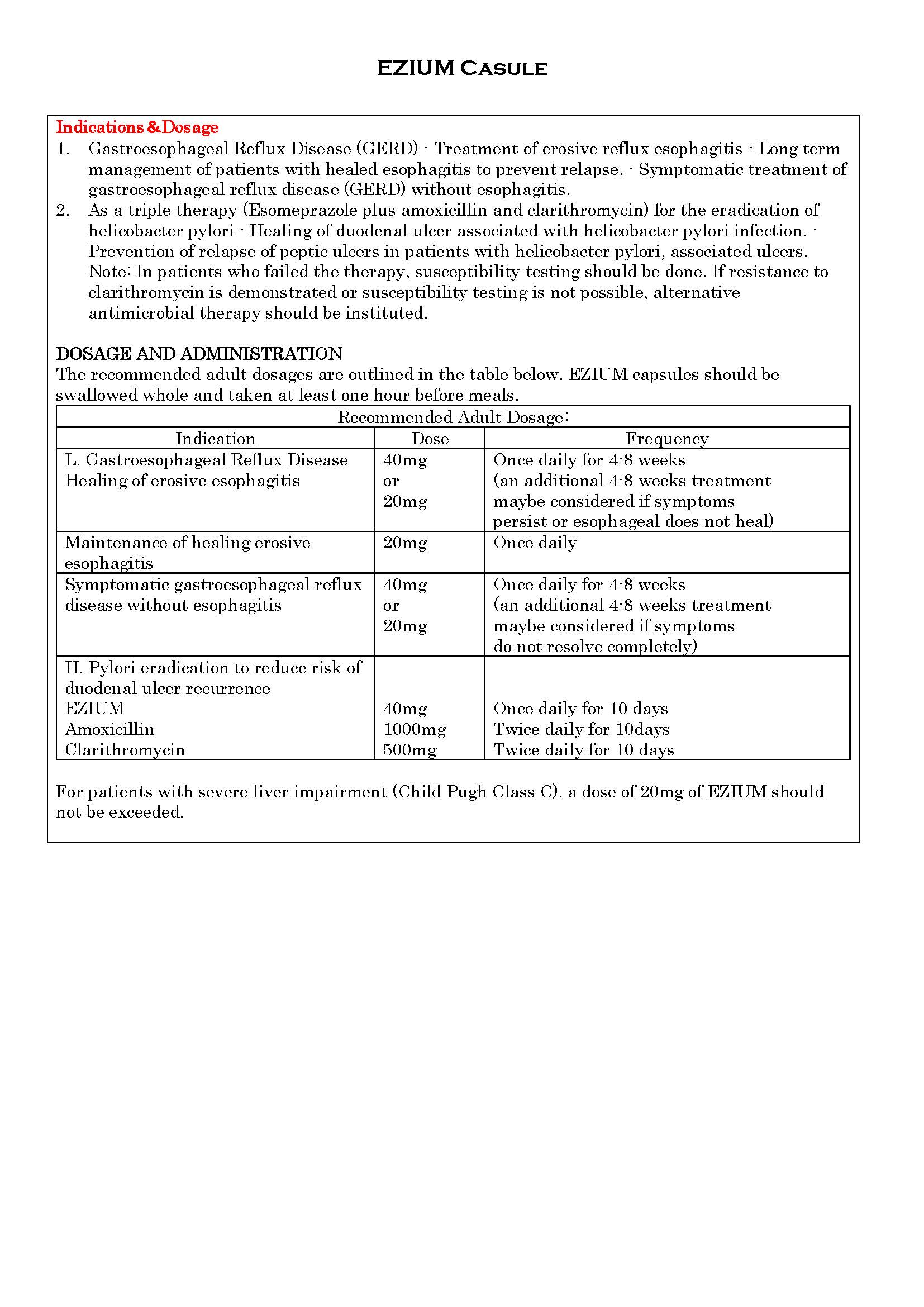
-
ហាមប្រើ
In patients with known hypersensitivity to drug or any component of the formulation or to substituted benzimidazoles.
-
ផលរំខាន
The following adverse drug reactions have been reported during therapy of esomeprazole. None found to be dose-related.
Common: Headache, abdominal pain, diarrhoea, flatulence, nausea/vomiting, constipation.
Uncommon: Dermatitis, pruritus, urticaria, dizziness, dry mouth.
Rare: Hypersensitivity reactions e.g. angioedema, anaphylactic reaction. The following adverse drug reactions have been observed for the racemate omeprazole and may occur with esomeprazole.
Central and peripheral nervous system:
Paraesthesia, somnolence, insomnia, vertigo. Reversible mental confusion, agitation, aggression, depression and hallucinations, predominantly in severely ill patents.
Endocrine: Gynecomastia.
Gastrointestinal: Stomatitis and gastrointestinal candidiasis.
Haematological: Leukopenia, thrombocytopenia, agranulocytosis and pancytopenia.
Hepatic: Increased liver enzymes, encephalopathy in patients with pre-existing severe liver disease, hepatitis with or without jaundice, hepatic failure.
Skin: Rash, photosensitivity, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN), alopecia.
Other: Malaise, hypersensitivity reactions e.g. fever, bronchospasm, interstitial nephritis. Increased sweating, peripheral oedema, blurred vision, taste disturbance & hyponatraemia.
-
អន្តរប្រតិកម្ម
In common with the use of other inhibitors of acid secretion or antacids, the absorption of ketoconazole and itraconazole can decrease due to decreased intragastric acidity during treatment with esomeprazole.
- Esomeprazole inhibits CYP2C19, the major esomeprazole metabolizing enzyme. Thus, when esomeprazole is combined with drugs metabolized by CYP2C19, such as diazepam, citalopram, imipramine, clomipramine, phenytoin etc, the plasma concentrations of these drugs may be increased and a dose reduction could be needed.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
There are no adequate and well-controlled studies in pregnant women. Esomeprazole should be used during pregnancy only if clearly needed.
Nursing Mothers
Because esomeprazole is likely to be excreted in human milk a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account importance of the drug to the mother due to the potential for serious adverse reactions in nursing infants from esomeprazole.
-
ការប្រុងប្រយ័ត្នជាពិសេស
General
In the presence of any alarming symptoms (e.g. significant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with esomeprazole may elevate symptoms and delay diagnosis. Patients on long-term treatment (particularly those treated for more than a year) should be kept under regular surveillance since the symptomatic response to therapy with esomeprazole does not preclude the gastric malignancy.
- Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole, of which esomeprazole is an enantiomer. Patient’s on-demand treatment should be instructed to contact their physician if their symptoms change in character.
- When prescribing esomeprazole for an on-demand therapy, the implications for interactions with other pharmaceuticals, due to fluctuating plasma concentrations of esomeprazole should be considered.
- When prescribing esomeprazole for eradication for helicobacter pylori infection possible drug interactions for other components in the triple therapy should be considered.
- Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrose-isomaltase insufficiency should not take this medicine.
-
សកម្មភាពឱសថ
Esomeprazole works by binding irreversibly to the H+/K+ ATPase in the proton pump. Because the proton pump is the final pathway for secretion of hydrochloric acid by the parietal cells in the stomach, its inhibition dramatically decreases the secretion of hydrochloric acid into the stomach and alters gastric pH.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
