CEFUROXIME AXETIL Tablet
ក្រុមហ៊ុនផលិតឱសថ:
LINCOLN PHARMACUETICALS LTD., India
- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
1. CEFUROXIME AXETIL Tablet 250mg:
Anhydrous cefuroxime 250mg
2. CEFUROXIME AXETIL Tablet 500mg:
Anhydrous cefuroxime 500mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
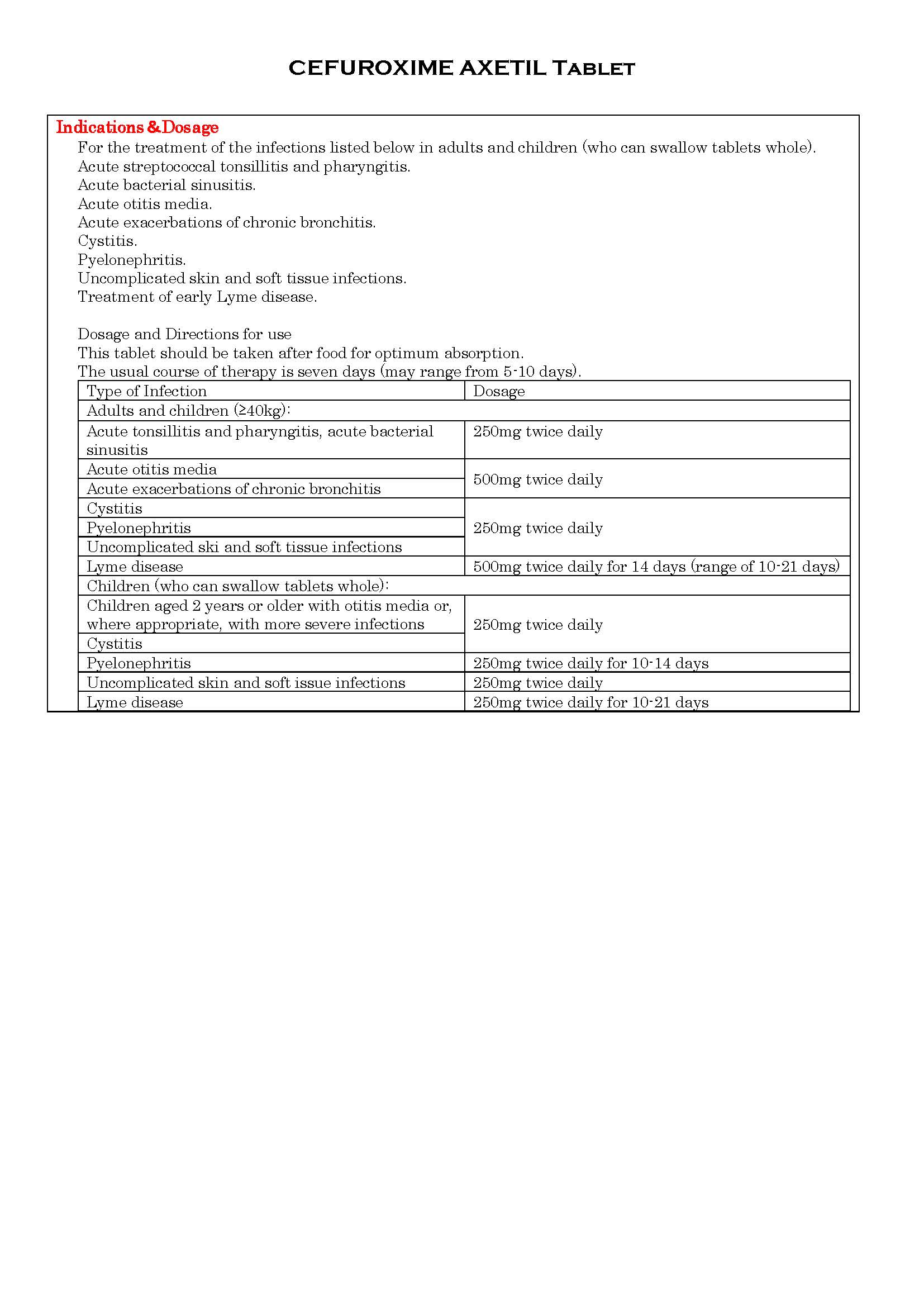
-
ហាមប្រើ
Hypersensitivity to cefuroxime, other cephalosporins or to any of the excipients.
History of severe hypersensitivity (e.g. anaphylactic reaction) to any other type of beta-lactam antibacterial agent (penicillins, monobactams and carbapenems).
-
ផលរំខាន
Gastrointestinal disorders: Diarrhoea, nausea, abdominal pain, vomiting, pseudomembranous colitis.
Nervous system disorders: Headache, dizziness, seizure.
Hepatobiliary disorders: Transient increases of hepatic enzyme levels, jaundice (predominantly cholestatic), and hepatitis.
Skin and subcutaneous tissue disorders: Skin rashes, urticaria, pruritus, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, angioneurotic oedema.
Blood and lymphatic system disorders: Increased prothrombin time, hemolytic anemia, leukopenia, pancytopenia, and thrombocytopenia.
Immune system disorders: Drug fever, serum sickness, anaphylaxis, Jarisch-Herxheimer reaction.
Infections and infestations: Candida overgrowth.
-
អន្តរប្រតិកម្ម
Drugs that reduce gastric acidity may result in a lower bioavailability of cefuroxime axetil compared with that of fasting state and tend to cancel the effect of postprandial absorption.
Cefuroxime axetil may affect the gut flora, leading to lower oestrogen reabsorption and reduced efficacy of combined oral contraceptives.
Cefuroxime is excreted by glomerular filtration and tubular secretion. Concomitant use of probenecid is not recommended.
Concurrent administration of probenecid significantly increases the peak concentration, area under the serum concentration time curve and elimination half-life of cefuroxime.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Pregnancy
There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Lactation
Because cefuroxime is excreted in human milk, consideration should be given to discontinuing nursing temporarily during treatment with cefuroxime axetil.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Hypersensitivity reactions: Special care is indicated in patients who have experienced an allergic reaction to penicillins or other beta-lactam antibiotics because there is a risk of cross-sensitivity. As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions have been reported. Caution should be used if cefuroxime is given to patients with a history of non-severe hypersensitivity to other beta-lactam agents.
Jarisch-Herxheimer reaction: The Jarisch-Herxheimer reaction has been seen following cefuroxime axetil treatment of Lyme disease. It results directly from the bactericidal activity of cefuroxime axetil on the causative bacterial of Lyme disease, the spirochaete Borrelia burgdorferi. Patients should be reassured that this is a common and usually self-limiting consequence of antibiotic treatment of Lyme disease.
Pediatric Use: There is no experience of using Cefuroxime axetil in children under the age of 3 months.
Renal impairment: The safety and efficacy of cefuroxime axetil in patients with renal failure have not been established. Cefuroxime is primarily excreted by the kidneys. In patients with markedly impaired renal function it is recommended that the dosage of cefuroxime should be reduced to compensate for its slower excretion. Cefuroxime is effectively removed by dialysis.
Hepatic impairment: There are no data available for patients with hepatic impairment. Since cefuroxime is primarily eliminated by the kidney, the presence of hepatic dysfunction is expected to have no effect on the pharmacokinetics of cefuroxime.
-
សកម្មភាពឱសថ
Cefuroxime axetil undergoes hydrolysis by esterase enzymes to the active antibiotic, cefuroxime. Cefuroxime inhibits bacterial cell wall synthesis following attachment to penicillin binding proteins (PBPs). This results in the interruption of cell wall (peptidoglycan) biosynthesis, which leads to bacterial cell lysis and death.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
