ZYRTEC Tablet
ក្រុមហ៊ុនផលិតឱសថ:
UCB Farchim SA Bulle, Switzerland
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
DKSH

- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Cetirizine 10mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់

-
ហាមប្រើ
Cetirizine is contraindicated in :
・hypersensitivity to any of constituents of this formulation, to hydroxyzine or to any piperazine derivatives,
・patients with severe renal impairment at less than 10 ml/min creatinine clearance.
-
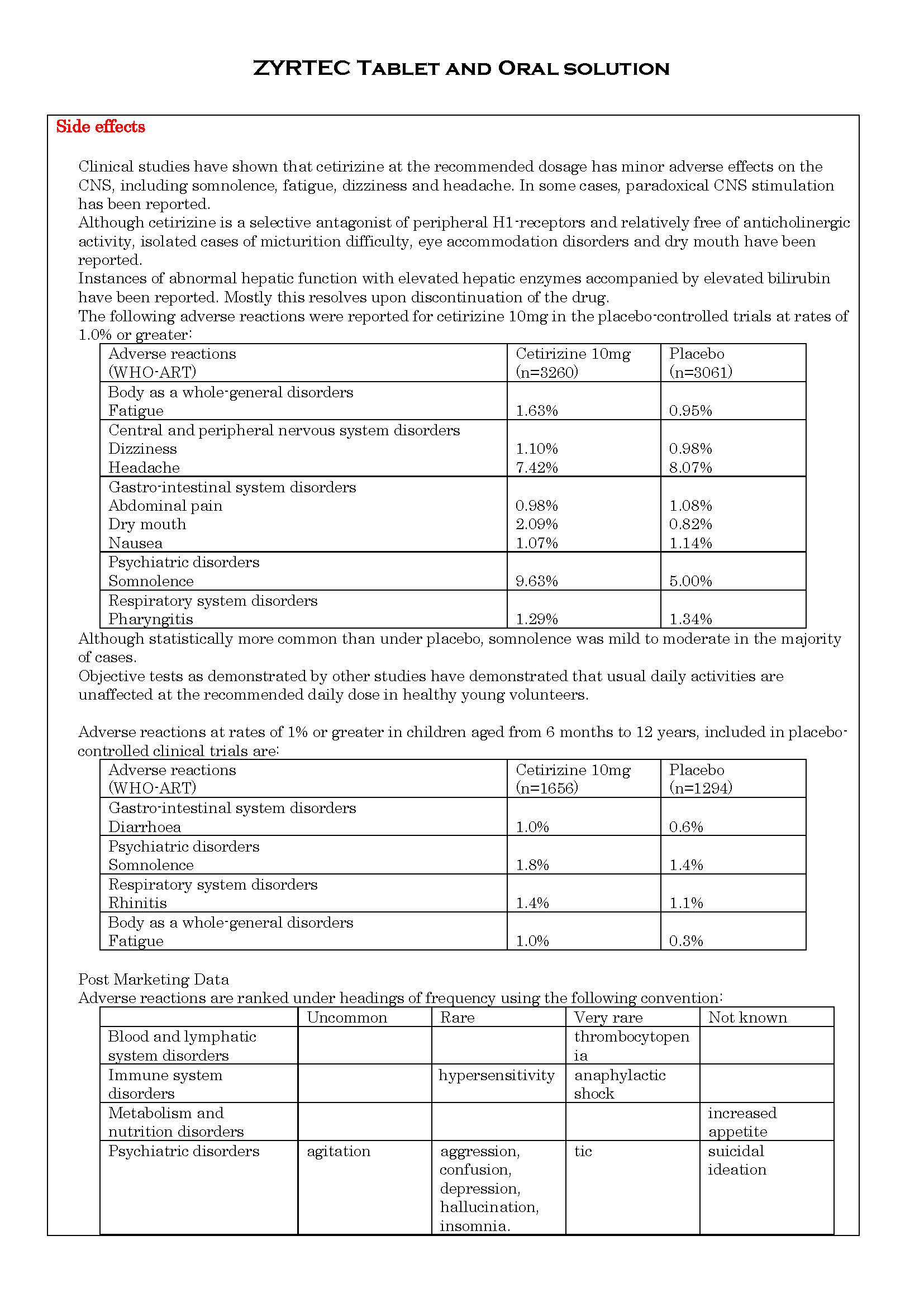
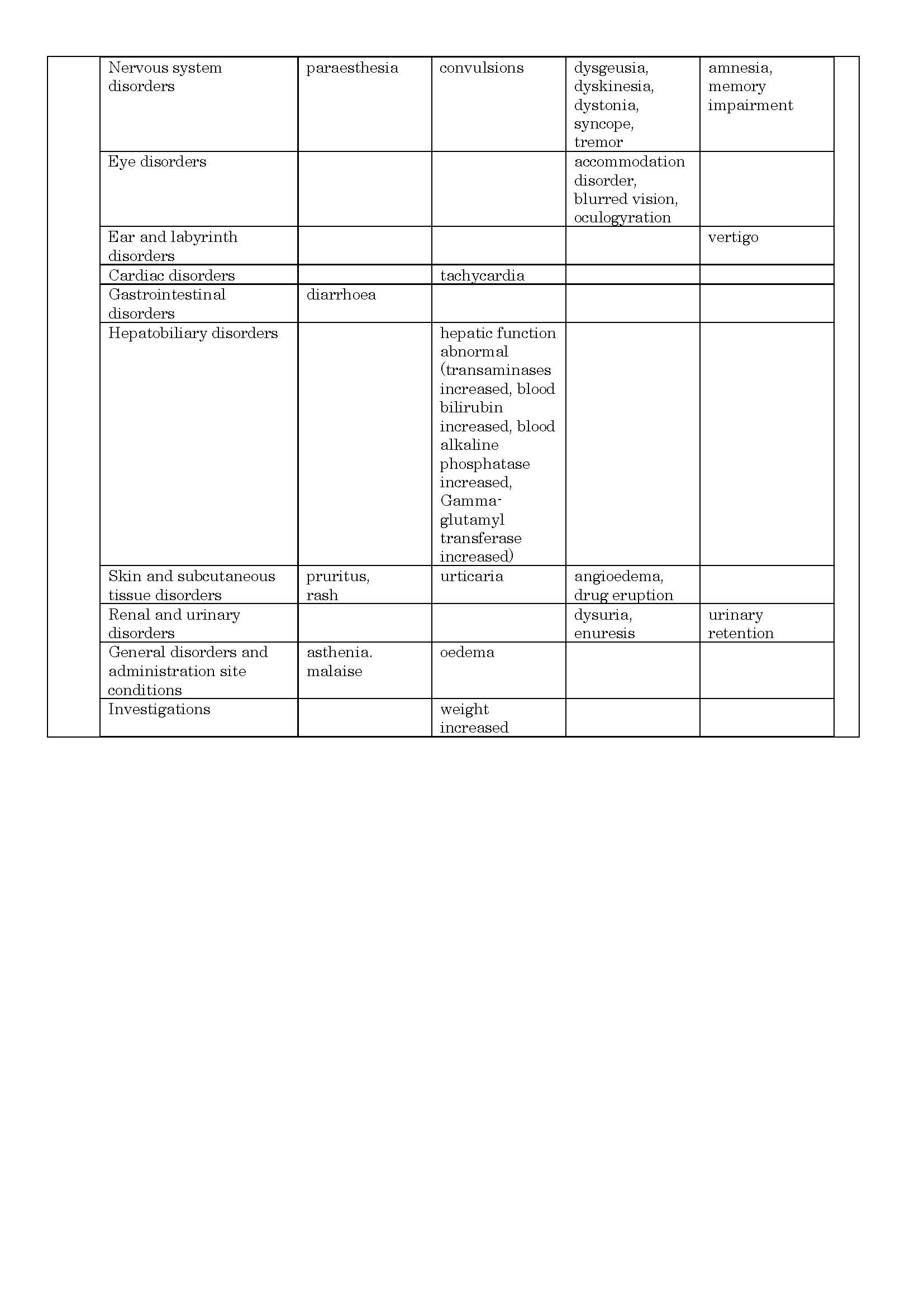
ផលរំខាន
-
អន្តរប្រតិកម្ម
Due to the pharmacokinetic, pharmacodynamic and tolerance profile of cetirizine, no interactions are expected with this antihistamine. Neither pharmacodynamic nor significant pharmacokinetic interaction was reported in drug-drug interactions studies performed, notably with pseudoephedrine or theophylline (400mg/body).
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
Fertility
There are no relevant data available.
Pregnancy
Caution should be exercised when prescribing to pregnant women.
For cetirizine very rare clinical data on exposed pregnancies are available. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development, parturition or postnatal development.
Lactation
Caution should be exercised when prescribing cetirizine to lactating women. Cetirizine is excreted in human milk at concentrations representing 25% to 90% of those measured in plasma, depending on sampling time after administration.
-
ការប្រុងប្រយ័ត្នជាពិសេស
Alcohol
At therapeutic doses, no clinically significant interactions have been demonstrated with alcohol (for a blood alcohol level of 0.5g/L). Nevertheless, precaution is recommended if alcohol is taken concomitantly.
Increased risk of urinary retention
Caution should be taken in patients with predisposition factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia) as cetirizine may increase the risk of urinary retention.
Patients at risk of convulsions
Caution in epileptic patients and patients at risk of convulsions is recommended.
Children
The use of the film-coated tablet formulation is not recommended in children aged less than 6 years since this formulation does not allow for appropriate dose adaptation. It is recommended to use a paediatric formulation of cetirizine.
The half-life of cetirizine was about 6 hours in children of 6-12 years and 5 hours in children 2-6 years.
Elderly, Renal/Hepatic impairment
See package insert.
Allergy skin tests
Allergy skin tests are inhibited by antihistamines and a wash-out period of 3 days is recommended before performing them.
Food
The extent of absorption of cetirizine is not reduced with food, although the rate of absorption is decreased.
Excipients
Lactose
Film-coated tablet, 10mg
This product contains lactose. Patients with rare hereditary problems of galactose intolerance, (the Lapp lactase deficiency or glucose-galactose malabsorption) should not take this medicine.
Sorbitol
Oral solution, 1mg/ml
This product contains sorbitol. Patients with rare hereditary problems of fructose intolerance should not take this medicine.
Sucrose
Oral solution, 1mg/ml
This product contains sucrose. Patients with rare hereditary problems of fructose intolerance, glucosegalactose malabsorption or sucrose-isomaltase insufficiency should not take this medicine.
Parabens
Oral solution, 1mg/ml
This product contains methylparahydroxybenzoate or propylparahydroxybenzoate, which may cause allergic reactions (possibly delayed).
-
សកម្មភាពឱសថ
Cetirizine, a human metabolite of hydroxyzine, is a potent and selective antagonist of peripheral H1-recptors. In vitro receptor binding studies have shown no measurable affinity for receptors other than H1-receptors.
Ex vivo experiments in mice have shown that systemically administered cetirizine does not significantly occupy the cerebral H1-receptors.
In addition to its anti-H1 effect, cetirizine was shown to display anti-allergic activities: at a dose of 10mg once or twice daily, it inhibits the late phase recruitment of inflammatory cells, notably eosinophils, in the skin and conjunctiva of atopic subjects submitted to antigen challenge, and the dose of 30mg/day inhibits the influx of eosinophils in the bronchoalveolar lavage fluid during a late-phase bronchial constriction induced by allergen inhalation in asthmatic subjects. Moreover, cetirizine inhibits the late-phase inflammatory reaction induced in chronic urticaria patients by intradermal administration of kallikrein. It also down-regulates the expression of adhesion molecules, such as ICAM-1 and VCAM-1, which are markers of allergic inflammation.
Studies in healthy volunteers show that cetirizine, at doses of 5 and 10mg strongly inhibits the wheal and flare reactions induced by very high concentrations of histamine into the skin. The onset of activity after a single 10mg dose occurs within 20 minutes in 50% of the subjects and within one hour in 95%. This activity persists for at least 24 hours after a single administration. In a 35-day study in children aged 5-12, no tolerance to the antihistaminic effect (suppression of wheal and flare) of cetirizine was found. When a treatment with cetirizine is stopped after repeated administration, the skin recovers its normal reactivity to histamine within 3 days.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
