OMNICEF Capsule
ក្រុមហ៊ុនផលិតឱសថ:
Interphil Laboratories, Inc., Philippines
ក្រុមហ៊ុនចែកចាយឱសថនៅប្រទេសកម្ពុជា:
DKSH

- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Cefdinir 100mg
-
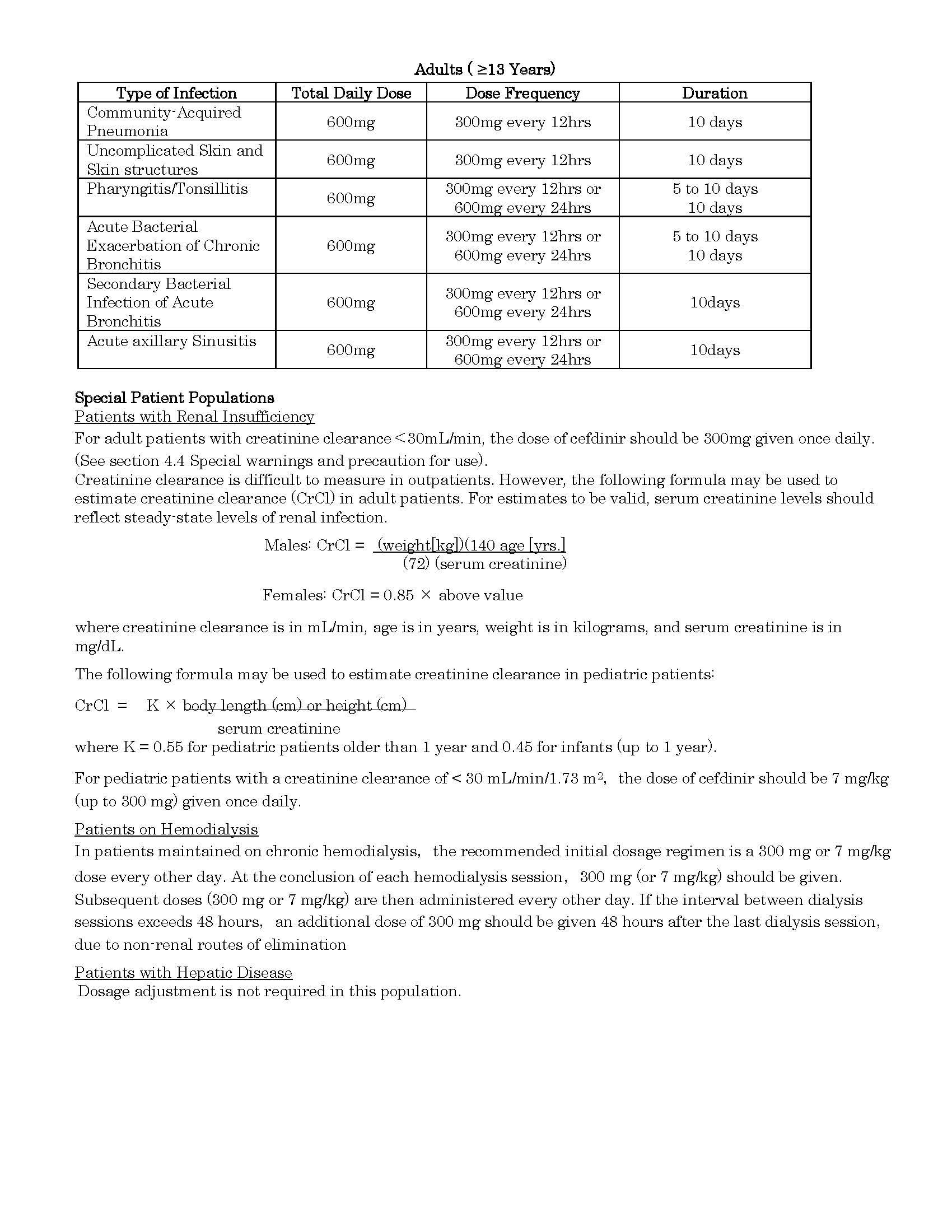
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់

-
ហាមប្រើ
ហាមប្រើចំពោះអ្នកងាយមានប្រតិកម្មជាមួយសារធាតុផ្សំណាមួយនៃឱសថនេះ ឬជាមួយក្រុមអង់ទីប៊ីយូទិចពួកCephalosporin។
Cefdinir is contraindicated for patients with known allergy to cefdinir or to the inactive ingredients of the product, or to the cephalosporin of antibiotics.
-
ផលរំខាន
ផលរំខានដែលគេរាយការណ៍ចំពោះអ្នកជំងឺ3841នាក់ដែលព្យាបាលដោយ Cefdinir៖
- ជំងឺឆ្លង៖ Candidasឆ្លងនៅយោនី និងឆ្លងនៅទ្វារមាស ជំងឺផ្សិតឆ្លងដោយមេរោគ Candidas
- វិបត្តិការបំលែង និងជីវជាតិ៖ អត់ឃ្លានអាហារ
- វិបត្តិផ្លូវចិត្ត៖ គេងមិនលក់
- វិបត្តិប្រព័ន្ធប្រសាទ៖ ឈឺក្បាល ធេងធោង ងងុយដេក
- វិបត្តិក្រពះពោះវៀន៖ រាក ចង្អោរ ឈឺពោះ ពិបាករំលាយអាហារ ហើមពោះ ក្អួត លាមកខុសធម្មតា ទល់លាមក ស្ងួតមាត់
- វិបត្តិស្បែក និងជាលិកាក្រោមស្បែក៖ កន្ទួល រមាស់លើស្បែក
- វិបត្តប្រព័ន្ធបន្តពូជ និងដោះ៖ ធ្លាក់ស
- វិបត្តិទូទៅ៖ អស់កំលាំង
ផលរំខានឱសថក្រោយដាក់លក់នៅលើទីផ្សារ៖
- ជំងឺឆ្លង៖ រលាកពោះវៀនធំ ជំងឺសួត
- ប្រព័ន្ធឈាម និងទឹករងៃ៖ ចំនួនគ្រាប់ឈាមថយចុះ ចំនួនកោសិកា granulocyteថយចុះ ដុំកំណកឈាមពេញក្នុងសរសៃឈាម ខ្វះឈាមក្រហមដោយសារមហារីកខួរឆ្អឹងក្រហម ចំនួនគ្រាប់ឈាមសថយចុះ ចំនួនប្លាកែតថយចុះ ជាំស្បែកដោយគ្មានមូលហេតុ ខ្វះឈាមក្រហមដោយសារបែកគ្រាប់ឈាម ភាពងាយកកឈាម ឬហូរឈាមខុសធម្មតា ជំងឺកកឈាម ចំនួនneutrophilថយចុះ។
- វិបត្តិប្រព័ន្ធភាពស៊ាំ៖ ប្រតិកម្មអាណាហ្វីឡាក់ទិច ភាពងាយមានប្រតិកម្ម
- វិបត្តិប្រព័ន្ធប្រសាទ៖ បាត់បង់ការដឹងខ្លួន ការមិនអាចត្រួតពិនិត្យចលនាដោយឆន្ទៈ
- វិបត្តិភ្នែក៖ ឈឺភ្នែកក្រហម
- វិបត្តិបេះដូង៖ ខ្សោយបេះដូង ក្រិនសាច់ដុំបេះដូង
- វិបត្តិសរសៃ៖ ហ្ស៊ុក សម្ពាធឈាមទាប ហូរឈាម
- វិបត្តិដង្ហើម សួត និងឆ្អឹងសន្ទះទ្រូង៖ ខ្សោយដង្ហើម ហើមបំពង់ខ្យល់ ជំងឺសួត កើតហឺត ជំងឺសួត មានអារម្មណ៍ថប់ដង្ហើម
- វិបត្តិក្រពះពោះវៀន៖ ហូរឈាមដោយសាររលាកពោះវៀន ហូរឈាមក្រពះ រាកមានឈាម ដំបៅក្រពះ លាមកមានឈាម រលាកពោះវៀន រលាកមាត់។Side effects
4.7 Effects on Ability to Drive and Use Machines
Caution should be taken when driving or using machinery while on this medication.
4.8 Undesirable Effects
Clinical Trials
Adult Patients
Capsules: 5093 adult and adolescent patients (3841 US and 1252 international) were treated with the recommended dose of cefdinir capsules (600 mg/day). No deaths or permanent disabilities were attributed to cefdinir. One hundred forty-seven of 5903 (3%) patients discontinued medication due to adverse events thought by the investigators to be associated with cefdinir therapy. The discontinuations were primarily for gastrointestinal disturbances,usually diarrhea or nausea; 19 of 5903 (0.4%) patients were discontinued due to rash thought related to cefdinir administration
The following adverse events were reported in 3841 patients treated with cefdinir in US trials.
Infections and infestations: Vulvovaginal candidiasis,Vaginal infection, Candidiasis
Metabolism and nutrition disorders: Decreased appetite
Psychiatric disorders: Insomnia
Nervous system disorders: Headache, Dizziness, Somnolence
Gastrointestinal disorders: Diarrhea,Nausea,Abdominal pain,Dyspepsia,Flatulence, Vomiting,Abnormal faeces, Constipation, Dry mouth
Skin and subcutaneous tissue disorders: Rash, Pruritus
Reproductive system and breast disorders: Genital discharge
General disorders and administration site conditions: Asthenia
Pediatric Patients
Suspension: 2289 pediatric patients (1783 US and 506 international) were treated with the recommended dose of cefdinir suspension (14 mg/kg/day). No deaths or permanent disabilities were attributed to cefdinir. Forty of 2289 (2%) patients discontinued mediation due to adverse events thought by the investigators to be associated with cefdinir therapy. The discontinuations were primary for gastrointestinal disturbances, usually diarrhea: 5 of 2289 (0.3%) patients were discontinued due to rash thought related to cefdinir administration.
The following adverse events were reported in 1783 cefdinir treated patients in US trials.
Infections and infestation: Skin candida, Vulvovaginal candidiasis, Vaginal infection
Blood and lymphatic system disorders; Leukopenia
Nervous system disorders: Hyperkinesia
Gastrointestinal disorders: Diarrhea, Nausea, Abdominal pain, Dyspepsia, Vomiting, Abnormal faces
Skin and subcutaneous tissue disorders: Rash, Rash maculo-papular
Investigations: Aspartate aminotransferase increased
Laboratory Value Changes
Adult Patients: The following laboratory value changes of possible clinical significance, irrespective of relationship to cefdinir therapy, were seen during trials.
Those that occurred with an incidence>1% sere:
Renal and urinary disorders: Haematuria, Leukocyturia
Investigations: Protein urine present, White blood cells urine positive, Gamma-glutamyltransferase increased, Lymphocyte count decreased, Lymphocyte count increased
Pediatric Patients: The following laboratory value changes of possible clinical significance, irrespective of relationship to cefdinir therapy, were seen during clinical trials. Those which occurred with an incidence>1% were:
Investigations: Lymphocyte count increased, Lymphocyte count decreased, Blood alkaline phosphatase increased, Blood bicarbonate decreased, Eosinophil count increased. Blood lactate dehydrogenase increased, Platelet count increased, Band neutrophil count increased, Band neutrophil count decreased, Protein urine present
Post-marketing Experience
In addition to the events reported during clinical trials,the following adverse events and altered laboratory tests,regardless of their relationship to the drug,have been reported during extensive post-marketing experience,beginning with approval in Japan in 1991.
Infections and infestations: Pseudomembranous colitis Pneumonia
Blood and lymphatic system disorders: Pancytopenia, Agranulocytosis, Disseminated intravascular coagulation, Aplastic anemia, Leukopenia, Thrombocytopenia, Granulocytopenia, Idiopathic thrombocytopenic purpura, Haemolytic anemia, Haemorrhagic diathesis, Coagulopathy, Neutropenia
Immune system disorders: Anaphylactic reaction, Hypersensitivity
Nervous system disorders: Loss of consciousness, Dyskinesia
Eye disorders: Conjunctivitis
Cardiac disorders: Cardiac failure, Myocardial infarction
Vascular disorders: Shock, Hypertension, Haemorrhage
Respiratory, thoracic and mediastinal disorders: Acute respiratory failure, Laryngeal oedema, Interstitial lung disease, Asthma, Eosinophilic pneumonia, Suffocation feeling
Gastrointestinal disorders: Enterocolitis Haemorrhagic, Upper gastrointestinal haemorrhage, Diarrhea
haemorrhagic, Peptic ulcer, Ileus, Melaena, Enterocolitis, Stomatitis
Hepatobiliary disorders: Hepatitis fulminant, Hepatic failure, Hepatitis acute, Cholestasis, Jaundice
Skin and subcutaneous tissue disorders: Toxic epidermal necrolysis, Stevens-Johnson syndrome,
Erythema multiforme, Dermatitis exfoliative, Erythema nodosum, Leukocytoclastic vasculitis
Musculoskeletal and connective tissue disorders: Rhabdomyolysis
Renal and urinary disorders: Renal failure acute, Nephropathy, Nephropathy toxic
General disorders and administration site conditions: Face oedema, Pyrexia, Chest pain
Investigations: Blood amylase increased
-
អន្តរប្រតិកម្ម
អន្តរកម្មឱសថ
- Antacids: លេប Cefdinir 300ម.ក្រ ជាមួយ Maalox TC 30ml បន្ថយអត្រាក្នុងឈាមរបស់ Cefdinir ចំនួន 44%។ គួរតែលេប Cefdinir 2ម៉ោងមុនឬក្រោយការលេប Antacids។
- Probenecid: ដូចអង់ទីប៊ីយូទិចក្រុម β-lactams ផ្សេងទៀត Probenecid រារាំងការបញ្ចេញចោល Cefdinir មកក្រៅតាមទឹកនោម នាំអោយកើនឡើងប្រហែល2ដងនូវ AUC កំហាប់ខ្ពស់បំផុត Cefdinir កើនឡើងនៅក្នុងប្លាស្មា 54% និងពន្យារការបញ្ចេញ half-life។
- ឱសថជាតិដែកឬអាហារមានជាតិដែក៖ ការប្រើដំណាលគ្នា Cefdinir ជាមួយអាហារបំប៉នមានជាតិដែកសំរាប់ព្យាបាល ឬវីតាមីនដែលមានជាតិដែក បន្ថយកម្រិតជ្រាបចូលក្នុង 80% និង 31% រៀងគ្នាតាមលំដាប់។ បើសិនអាហារបំប៉នមានជាតិដែកត្រូវការពេលព្យាបាលដោយ Cefdinir ត្រូវលេបCefdinir 2ម៉ោងមុនឬក្រោយការប្រើអាហារបំប៉នមានជាតិដែក។Antacids
Iron-Supplements and Foods Supplemented With Iron
Laboratory Tests
A false-positive reaction for ketones in the urine may occur with tests using nitroprusside, but not with those using nitroferricyanide. The administration of cefdinir may result in a false-positive reaction for glucose in the urine using some diagnostic test kits, Benedict’s solution, of Fehling’s solution. It is recommended that glucose tests based on enzymatic glucose oxidase reactions be used. Cephalosporins are known to occasionally induce a positive direct Coombs test.
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
・ស្ត្រីមានផ្ទៃពោះ៖ ដោយសារគ្មានទិន្នន័យគ្រប់គ្រាន់អំពីការប្រើឱសថនេះលើស្ត្រីមានផ្ទៃពោះ។ ដោយសារការសិក្សាលើសត្វមិនអាចព្យាករណ៍បានជានិច្ចលើមនុស្ស ឱសថនេះអាចប្រើបានពេលមានផ្ទៃពោះតែនៅពេលណាដែលផលចំណេញមានច្រើនលើសហានិភ័យតែប៉ុណ្ណោះ។
・ស្ត្រីបំបៅដោះកូន៖ បន្ទាប់ពីប្រើកម្រិត 600ម.ក្រ Cefdinir មិនត្រូវបានគេរកឃើញមានក្នុងទឹកដោះម្ដាយទេ។Pregnancy: There are no adequate and well-controlled studies in pregnant women. (See Carcinogenesis, Mutagenesis, Impairment of Fertility.) Because animal reproduction studies are not always predictive of human response, cefdinir should be used during pregnancy only if the potential benefits clearly outweigh the risks. Cefdinir has not been studied for use during labor and delivery.
Lactation: Following administration of single 600mg doses, cefdinir was not detected in human breast milk.
-
ការប្រុងប្រយ័ត្នជាពិសេស
ការប្រុងប្រយ័ត្ន
- ប្រតិកម្មអាល្លែកហ្ស៊ី៖ មុនពេលប្រើ Cefdinir ត្រូវសួរសំណួរដោយប្រុងប្រយ័ត្នដើម្បីកំណត់ថាតើអ្នកជំងឺធ្លាប់ងាយមានប្រតិកម្មជាមួយ Cefdinir ក្រុម β-lactams ផ្សេងទៀត (Cephalosporin), Penicillins ឬ ឱសថផ្សេងទៀត។
- ការលូតលាស់មេរោគផ្សេងទៀត៖ ការប្រើក្រុមអង់ទីប៊ីយូទិចដែលមាន spectre ធំទូលាយដូចជា Cefdinir វាធ្វើអោយប្រែប្រួល flore នៅពោះវៀន និងអាចធ្វើអោយ clostridia ដុះលូតលាស់ច្រើន។ ការសិក្សាបង្ហាញថាជាតិពុលដែលបង្កដោយ clostridium difficile ជាមូលហេតុចំបងនៃជំងឺរលាកពោះវៀនធំដោយសារការប្រើក្រុមអង់ទីប៊ីយូទិច រួមទាំង ការរលាកពោះវៀនធំ pseudomembranous។
- ជំងឺផ្នែកកុមារ៖ សុវត្ថិភាព និងប្រសិទ្ធិភាព ចំពោះក្មេងទើបកើត និងក្មេងអាយុក្រោយ6ខែមិនត្រូវបានគេសិក្សានៅឡើយ។Special Precaution
Hypersensitivity
Before cefdinir is administered, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to cefdinir, other-lactams (cephalosporins), penicillis or other drugs. If cefdinir is to be given to penicillin-sensitive patients, caution should be exercised because cross-hypersensitivity among-lactam antibiotics has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to cefdinir occurs, the drug should be discontinued. Serious acute hypersensitivity reactions may require treatment with epinephrine and other emergency measures, including oxygen, intravenous fluids, intravenous antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated.
Bacterial Overgrowth
Treatment with broad-spectrum antibiotics, including cefdinir, alters the normal floral of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of antibiotic-associated colitis, including cefdinir, therefore, it is important to consider this diagnosis in patients who develop diarrhea in association with the use of antibiotics. Symptoms of pseudomembranous colitis may occur during or after discontinuation of antibiotic treatment and can range in severity from mild to life-threatening.
After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids, electrolytes, protein supplementation, and treatment with antibacterial drug clinically effective against Clostridium difficile.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefdinir, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C.difficile.
C.difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C.difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. As with other broad-spectrum antibiotics, prolonged treatment may result in the possible emergence and overgrowth of resistant organisms. Careful observation of the patient is essential. If super infection occurs during therapy, appropriate therapy should be administered.
As with all broad-spectrum antibiotics, cefdinir should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
In patients with transient of persistent renal insufficiency (creatinine clearance <30 mL/min), the total daily use of cefdinir should be reduced because high and prolonged serum antibiotic concentrations can occur following recommended doses.( See section 4.2 posology and method of administration)
Diabetic patients and their caregivers should be made aware that cefdinir oral suspension contains 2.9g of sucrose per 5mL.
If iron supplements are required during cefdinir therapy, cefdinir should be taken at least 2 hours before or after the iron supplement.
Cefdinir suspension can be administered with iron-fortified infant formula.
Some antacids interfere with the absorption of cefdinir. If an antacid is required during cefdinir therapy, cefdinir should be taken at least two hours before or after the antacid.
-
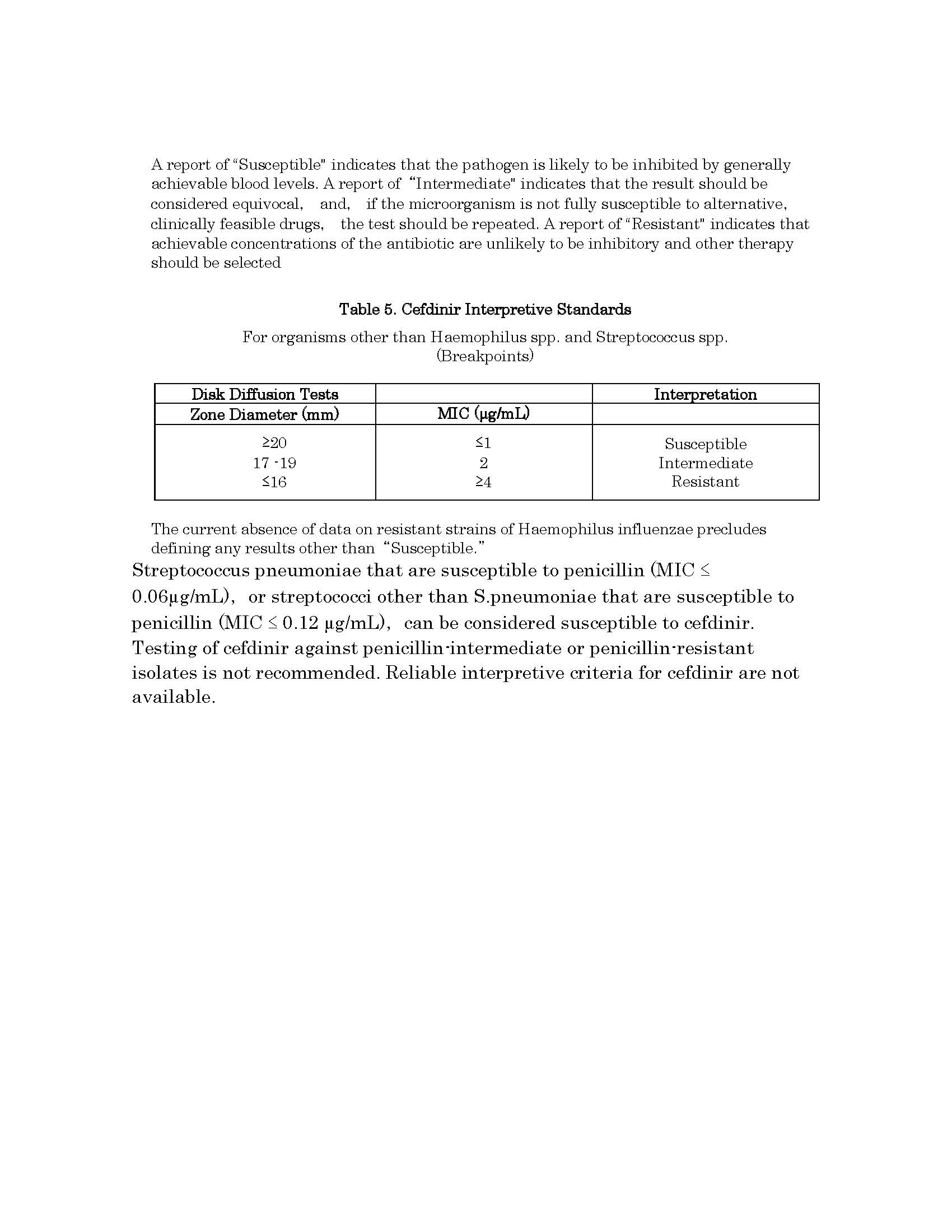
សកម្មភាពឱសថ

*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
