JAPROLOX Tablet
ក្រុមហ៊ុនផលិតឱសថ:
DAIICHI SANKYO PROPHARMA CO.,LTD., Japan

- សារធាតុសកម្ម
- ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
- ហាមប្រើ
- ផលរំខាន
- អន្តរប្រតិកម្ម
- ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
- ការប្រុងប្រយ័ត្នជាពិសេស
- សកម្មភាពឱសថ បរិយាយប័ណ្ណឱសថ
-
សារធាតុសកម្ម
Loxoprofen 60mg
-
ប្រសិទ្ធិភាពព្យាបាល និង កម្រិតប្រើប្រាស់
This drug has anti-inflammatory and analgesic effects on the following diseases and symptoms: Chronic articular rheumatism, osteoarthritis, Lumbago, periarthritis of the shoulder and Shoulder-arm-neck syndrome.
This drug relieves pain and inflammation after operation, trauma and tooth extraction.
Dosage
For general use, 60mg are orally administered to adults 3 times a day.
For single administration, 60-120mg are orally given. The dosages should be adjusted according to age and symptoms.
-
ហាមប្រើ
1. Patients with peptic ulcers
2. Patients with severe hematological abnormalities
3. Patients with severe hepatic dysfunction
4. Patients with severe renal dysfunction
5. Patients with severe cardiac function failure
6. Patients with a history of hypersensitivity to any of the components of this product
7. Patients with a history of aspirin-induced asthma
8. Women in the late stages of pregnancy
-
ផលរំខាន
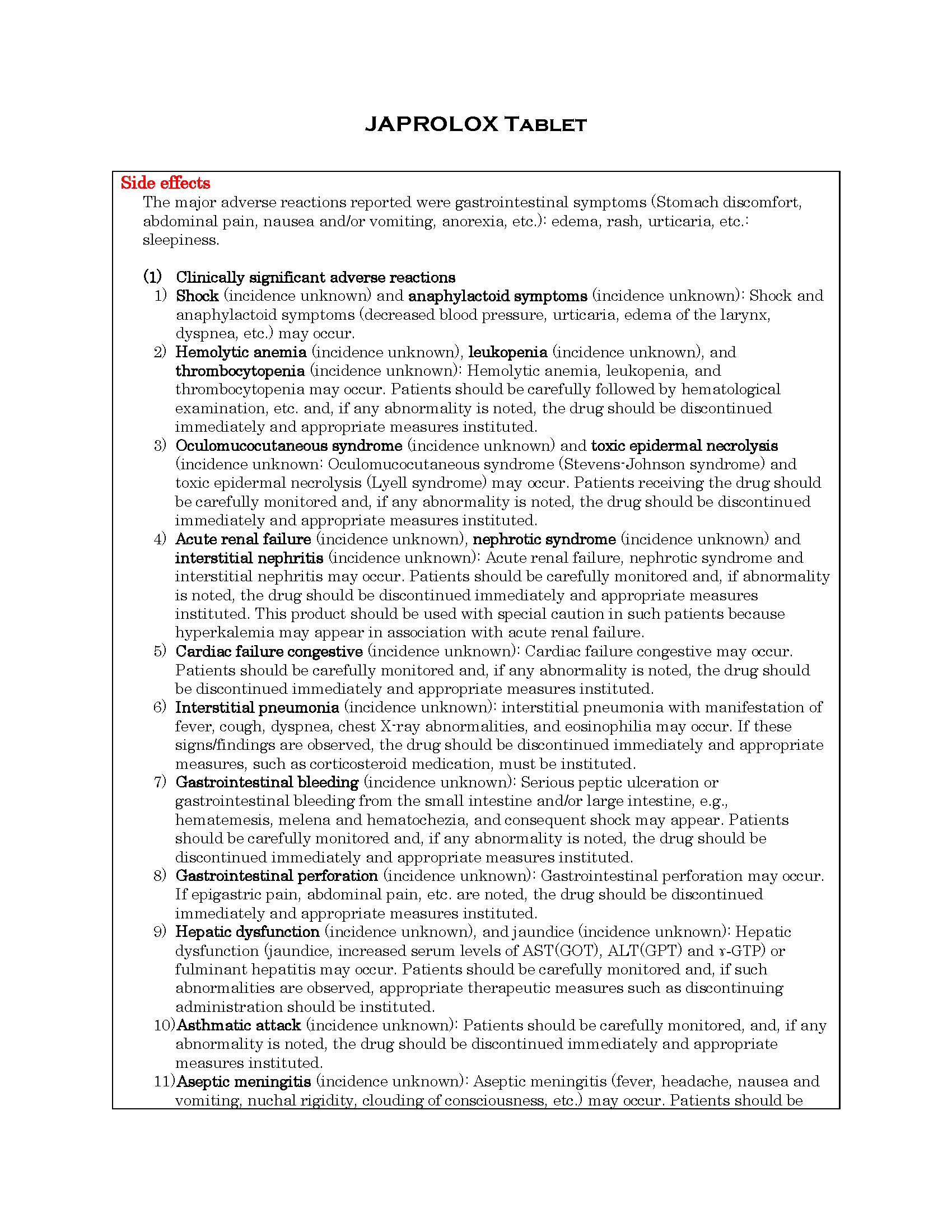
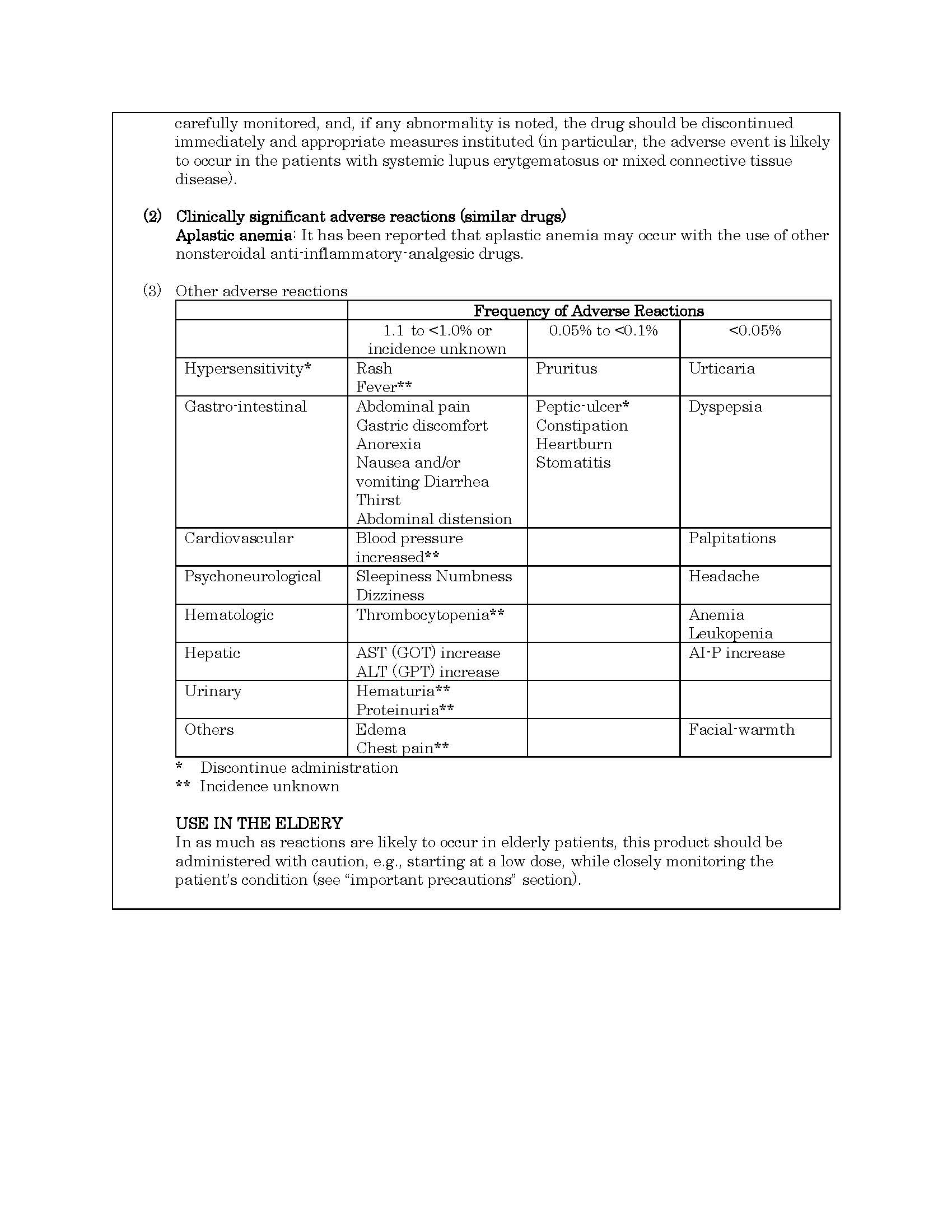
-
អន្តរប្រតិកម្ម
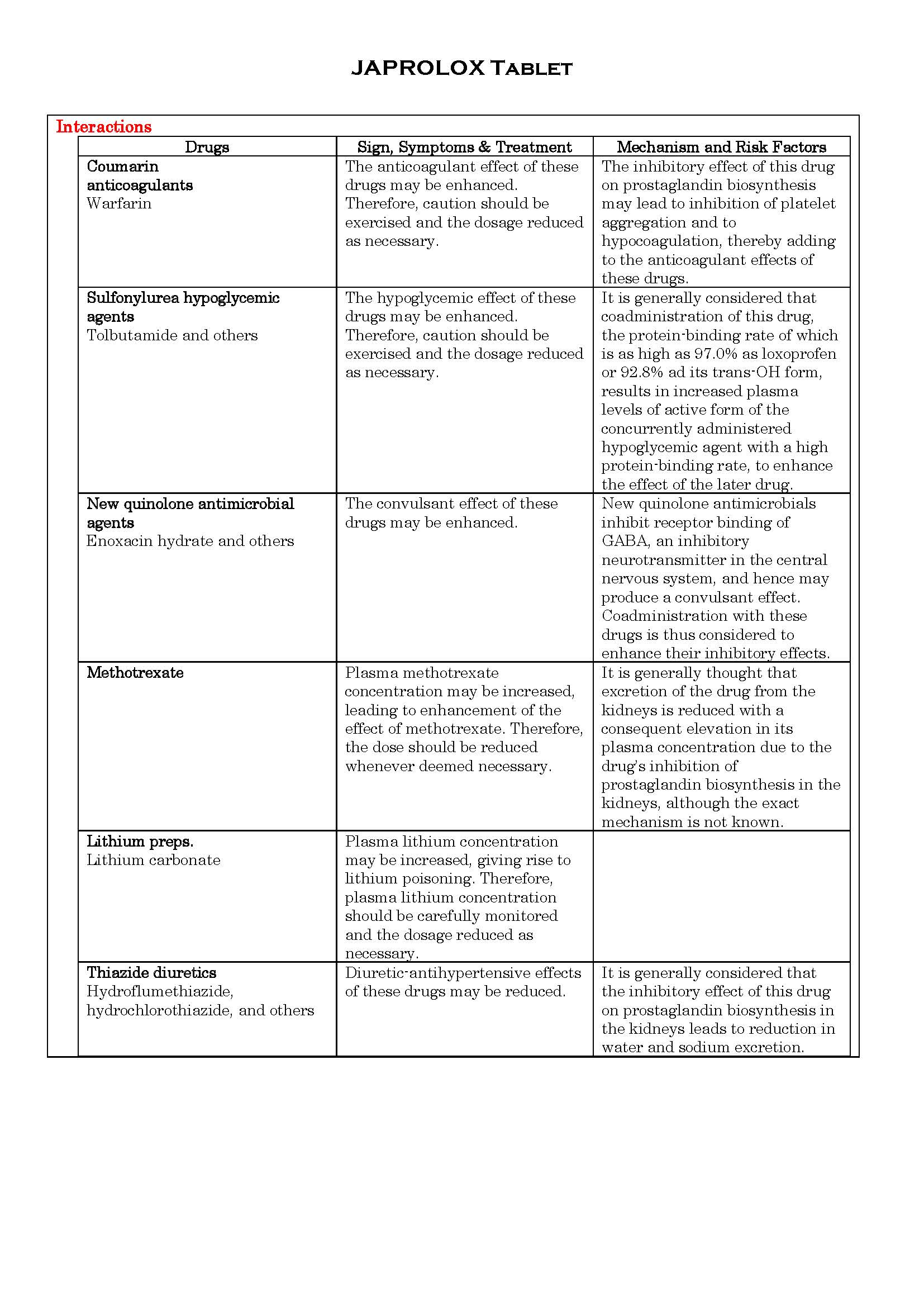
-
ស្ត្រីមានផ្ទៃពោះ និង ស្ត្រីបំបៅដោះកូន
1) This product should be administered to women who are or are possibly only when the anticipated therapeutic benefits are considered to outweigh any potential risk. (The safety of this product in pregnant women has not been established.)
2) This drug should not be used in women in the late stages of pregnancy. (Delayed parturition has been reported in an animal study (in rats).)
3) Fetal arterial vasoconstriction has been reported in a study on rats receiving the drug in the late stages of gestation.
4) Administration of this drug to nursing mothers should be avoided. If administration of this drug is judged to be essential, nursing should be discontinued. (Animal studies (in rats) have demonstrated that loxoprofen is secreted in breast milk.)
-
ការប្រុងប្រយ័ត្នជាពិសេស
1. Patients with a history of peptic ulcers (since the use of this product may cause recurrence of ulceration).
2. Patients with peptic ulcer associated with chronic use of NSAIDs whose clinical condition required long-term administration of this product and who are currently on misoprostol therapy. (This drug must be administered with care while closely monitoring the clinical condition of patients receiving this drug continuously, because peptic ulcers may be refractory to treatment with misoprostol, which is indicated for NSAIDs-induced peptic ulceration).
3. Patients with or with a history of blood abnormalities (since adverse reaction such as hemolytic anemia are prone to occur).
4. Patients with or with a history of hepatic dysfunction (because exacerbation or recurrence of the liver dysfunction may occur).
5. Patients with or with a history a renal dysfunction (since adverse reactions such as edema, proteinuria, serum creatinine elevation or hyperkalemia may occur).
6. Patients with cardiac dysfunction (see “CONTRAINDICATIONS”).
7. Patients with a history of hypersensitivity.
8. Patients with bronchial asthma (as the disease state may be exacerbated).
9. Patients with colitis ulcerative (as the disease state may be exacerbated).
10. Elderly subjects (see section on “Use in the Elderly”).
11. Patients with Crohn’s disease (as the disease state may be exacerbated).
-
សកម្មភាពឱសថ
relatively lower toxicity to the gastrointestinal tract than other non-steroidal analgesic and anti-inflammatory drugs because it is the prodrug showing the effect after absorption from the gastrointestinal tract and active metabolites.
The mode of action of this drug is prostaglandin biosynthesis inhibition and its side of action is cyclooxygenase.
After oral administration, this tablet is rapidly absorbed from the digestive tract in the unchanged from causing weak irritation to the stomach mucosa, and then rapidly converted to an active metabolite, the trans-alcohol from (in SRS configuration) which potently inhibit prostaglandin biosynthesis.
*ព័ត៌មានឱសថត្រូវបានរៀបរៀងដោយ អ៊ីម៉ាតុគឹ មេឌីក (ខេមបូឌា) ដោយផ្អែកលើប្រភពព័ត៌មានខាងក្រោម។ សម្រាប់ព័ត៌មានលម្អិត សូមស្វែងរកនៅក្នុងក្រដាសព័ត៌មាននៃឱសថនីមួយៗ ឬ សាកសួរទៅកាន់ក្រុមហ៊ុនឱសថឬតំណាងចែកចាយនៃឱសថនីមួយៗ។
ប្រភពព័ត៌មាន៖
- ក្រដាសព័ត៌មាននៃឱសថសម្រាប់អ្នកជំនាញវេជ្ជសាស្ត្រដែលប្រើប្រាស់នៅប្រទេសជប៉ុន (Pharmaceutical and Medical Devices Agency, Pmda): https://www.pmda.go.jp
- ព័ត៌មានសង្ខេបនៃឱសថសម្រាប់អ្នកជំងឺដែលប្រើប្រាស់នៅប្រទេសជប៉ុន: http://www.rad-ar.or.jp
